VSEPR Theory: Postulates, Predicting Shapes of Molecules
– In this subject, we will discuss the VSEPR Theory: definition, Postulates, limitations, and Predicting Shapes of Molecules.
VSEPR Theory
– The Lewis structure of a molecule tells us the number of pairs of electrons in the valence shell of the central atom.
– These electron pairs are subject to electrostatic attraction between them.
– On this basis, R.G. Gillespie (1970) proposed a theory called the Valence-Shell Electron Pair Repulsion or VSEPR (pronounced as ‘Vesper’) theory.
– VSEPR Theory states that: The electron pairs (both lone pairs and shared pairs, surrounding the central atom will be arranged in space as far apart as possible to minimise the electrostatic repulsion between them.
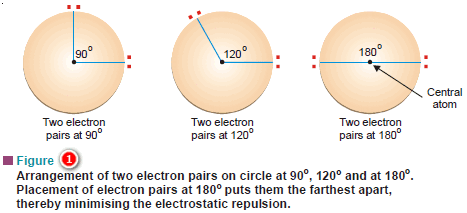
– Let us consider the simplest case of an atom with two electron pairs.
– We wish to place the electron pairs on the surface of a sphere such that they will be as far apart as possible to minimise repulsion between them.
– Fig (1) illustrates it by showing some possible placements of the two electron pairs.
– The arrangement in which the electron pair-central atom-electron pair angles is 180º, makes the electron pairs farthest apart.
– This arrangement is called linear because the electron pairs and the central atom are in a straight line.
– VSEPR theory is a simple but remarkably powerful model for predicting molecular geometries and bond angles.
– While working out the shapes of molecules from this theory, it must be remembered:
(1) Multiple bonds behave as a single electron-pair bond for the purpose of VSEPR. They represent a single group of electrons.
(2) Order of repulsions between lone pair and lone pair (lp-lp), lone pair and bonding pair (lp-bp), and bonding pair and bonding pair (bp-bp) is:
lp – lp > > lp – bp > bp – bp
– When a molecule has lone pairs of electrons, the bonding electron pairs are pushed closer and thus the bond angle is decreased.
– Now we proceed to work out the shapes of some common molecules with the help of VSEPR theory.
(1) Linear Molecules according to VSEPR Theory
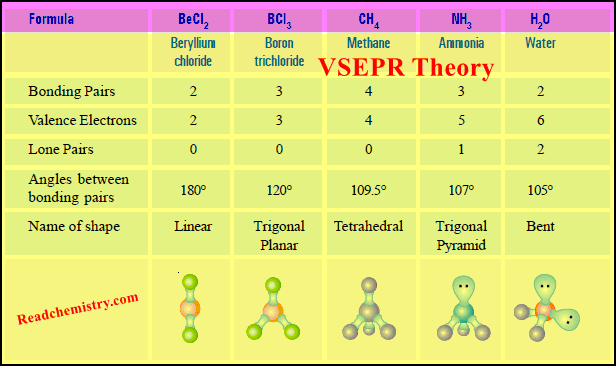
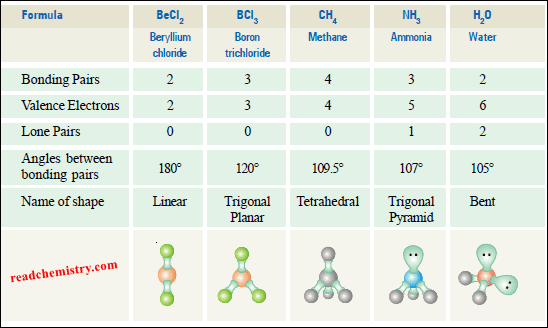
(a) Beryllium chloride, BeCl2
– It has the Lewis structure:
– The central atom Be has two bonding electron pairs and no unshared electron.
– According to VSEPR theory, the bonding pairs will occupy positions on opposite sides of Be forming an angle of 180º.
– An angle of 180º gives a straight line. Therefore, BeCl2 molecule is linear.
– In general, all molecules as A–B–A which have only two bonds and no unshared electrons are linear.
(b) Carbon dioxide, CO2
– It has the following structure:
– The central C atom has no unshared electron.
– We know that a double bond counts the same as a single bond in the VSEPR model.
– Thus CO2 is a linear molecule.
– Similarly, it can be shown that hydrogen cyanide (H – C ≡ N) and acetylene (H – C ≡ C – H) are linear molecules.
(2) Trigonal Planar Molecules according to VSEPR Theory
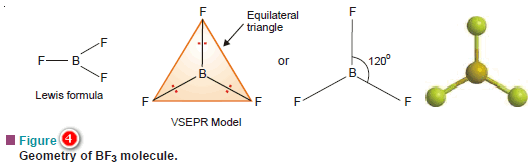
(a) Boron trifluoride, BF3
– Its Lewis structure shown that the central atom B has three bonding electron pairs and no unshared electrons.
– VSEPR theory says that the three bonding electron pairs will be as far apart as possible.
– This can be so if these electron pairs are directed to the corners of an equilateral triangle.
– Thus VSEPR model of BF3 molecule has three F atoms at the corners of the triangle with B atom at its centre.
– All four atoms (three F and one B) lie in the same plane.
– Therefore, the shape of such a molecule is called trigonal planar.
– The bond angle is 120º.
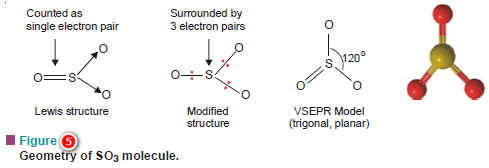
(b) Sulphur trioxide, SO3
– In the Lewis structure of SO3
– The central S atom is joined with two O atoms by covalent bonds.
– The third O atom is joined with S by a double bond.
– But a double bond is counted as a single electron pair for the purpose of the VSEPR model.
– Therefore, in effect, S has three electron pairs around it.
– Thus like BF3, SO3 has trigonal planar geometry.
(3) Tetrahedral Molecules according to VSEPR Theory
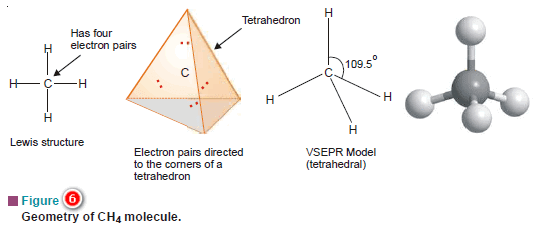
(a) Methane, CH4
– The Lewis structure of methane shows that the central C atom has four bonding electron pairs.
– These electron pairs repel each other and are thus directed to the four corners of a regular tetrahedron.
– A regular tetrahedron is a solid figure with four faces which are equilateral triangles.
– All bond angles are 109.5º.
– Similarly, CCl4 in which the central C atom is bonded to four other atoms by covalent bonds has tetrahedral shape.
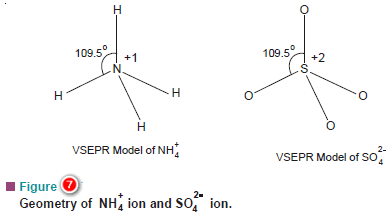
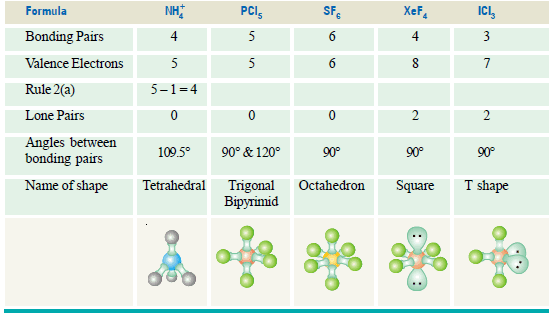
(b) Ammonium ion, NH4+, and Sulphate ion SO4-2
– The N atom in NH4+ and S atom in SO4-2 have four electron pairs in the valence shell.
– These are directed to the corners of a tetrahedron for maximum separation from each other.
– Thus both NH4+ and SO4-2 have tetrahedral shape.
(4) Pyramidal Molecules according to VSEPR Theory
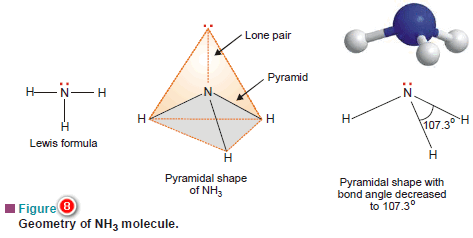
(a) Ammonia molecule, NH3
– The Lewis structure of NH3 shows that the central N atom has three bonding electrons and one lone electron pair.
– The VSEPR theory says that these electron pairs are directed to the corners of a tetrahedron.
– Thus we predict that H–N–H bond angle should be 109.5º.
– But the shape of a molecule is determined by the arrangement of atoms and not the unshared electrons.
– Thus, if we see only at the atoms, we can visualise NH3 molecule as a pyramid with the N atom located at the apex and H atoms at the three corners of the triangular base.
– According to VSEPR theory, a lone pair exerts greater repulsion on the bonding electron pairs than the bonding pairs do on each other.
– As a result, the bonds of NH3 molecule are pushed slightly closer.
– This explains why the observed bond angle H–N–H is found to be 107.3º instead of 109.5º predicted from tetrahedral geometry
– All molecules in which the N atom is joined to three other atoms by covalent bonds, have pyramidal shape.
– For example, amines RNH2, R2NH and R3N have pyramidal shape.
(b) Phosphorus trichloride, PCl3
– The structural formula indicates that the central phosphorus atom has three bonding electron pairs and one lone electron pair.
– Thus, like NH3 it has a pyramidal shape and the observed bond angle Cl–P–Cl is 100º.
(5) Bent or Angular Molecules according to VSEPR Theory
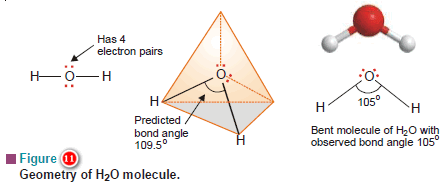
(a) Water, H2O
– In the structural formula of H2O, the O atom is bonded to two H atoms by covalent bonds and has two lone pairs.
– Thus O is surrounded by two bonding electron pairs and two unshared electron pairs.
– VSEPR theory says that to secure maximum separation between them, the four electron pairs are directed to the corners of a tetrahedron.
– If we look at the three atoms (and ignore the unshared pairs), the atoms HOH lie in the same plane and the predicted bond angle is 109.5º.
– But with two unshared pairs repelling the bonding pairs, the bond angle is compressed to 105º, the experimental value.
– Thus the H2O molecule is flat and bent at an angle at the O atom.
– Such a molecule is called a bent molecule or angular molecule.
(b) Sulphur dioxide, SO2
– The Lewis structure of SO2 is given below.
– The S atom is bonded to one O by a double bond and to the other O by a single bond.
– It has an unshared electron pair.
– In the VSEPR model a double bond is counted as a single electron pair.
– That way, the S atom is surrounded by three electron pairs, two bonding pairs and one unshared pair.
– For maximum separation, the three electron pairs are directed to the corners of an equilateral triangle.
– The predicted bond angle is 120º.
– But with the unshared electron pair repelling the bonding electron pairs, the bond angle is actually reduced somewhat.
– Thus SO2 has a planar bent molecule with an observed bond angle 119.5º.
Summary: shapes of molecules
– The directional nature of covalent bonds is shown in the diagrams of molecules above.
– The shape of the methane molecule is tetrahedral because the four bonding pairs of electrons repel each other equally, and the equilibrium position of all four bonding electron pairs is tetrahedral.
Predicting Shapes of Molecules according to VSEPR Theory
– It is possible to work out the shape of a small molecule that has the formula XYn by applying a few simple rules.
– We will use ammonia as an example to illustrate the idea.
Rule 1:
– First, find the number of bonding pairs of electrons in the molecule.
– The number of bonding pairs of electrons in the molecule NH3 can be seen in the formula.
– There must be three bonding pairs of electrons holding the three hydrogens onto the nitrogen.
Rule 2:
– Find the number of valence electrons (electrons in the outer energy level) on an atom of the central atom (The one of which there is only one.)
– Nitrogen is in group V, so nitrogen has five electrons in the outer energy level.
Rule 3:
– Find the number of lone pairs on the central atom by subtracting the number of bonding pairs (3) from the valence electrons (5) to find the number of electrons (2) that will make up lone pairs of electrons.
– Divide this number by 2 to find the number of lone pairs, 2/2 = 1.
Rule 4:
– Distribute all the electron pairs around the central atom and learn the angles they will make from molecules with no lone pairs.
Rule 5:
– Learn that the repulsion between lone pairs of electrons is greater than the repulsion between bonding pairs, and subtract 2o from the bond angles for every lone pair.
Rule 6:
– Learn the names of the shapes.
– The shapes are named from the position of the atoms and not the position of the orbitals.
– There is one more rule to learn, and it concerns the shape of polyatomic ions.
– Rule 2(a): If the molecule is an ion, e.g. ammonium (NH4+), subtract 1 from the number of valence electrons for every + charge on the ion and add 1 to the valence number for every – charge, then proceed as before.
Some more Examples of molecules
Limitations of VSEPR Theory
Some significant limitations of the VSEPR theory include
(1) This theory fails to explain isoelectronic species (i.e., elements having the same number of electrons).
– The species may vary in shape, despite having the same number of electrons.
(2) The VSEPR theory does not shed any light on the compounds of transition metals.
– The structure of several such compounds cannot be correctly described by this theory.
– This is because the VSEPR theory does not take into account the associated sizes of the substituent groups and the lone pairs that are inactive.
(3) Another limitation of the VSEPR theory is that it predicts that halides of group 2 elements will have a linear structure, whereas their actual structure is a bent one.