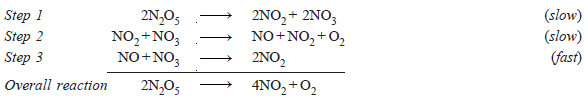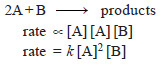– we will discuss the Molecularity of a reaction and the Differences Between Order and Molecularity.
Molecularity of a reaction
– Chemical reactions may be classed into two types :
(a) Elementary reactions
(b) Complex reactions
– An elementary reaction is a simple reaction which occurs in a single step.
– A complex reaction is that which occurs in two or more steps.
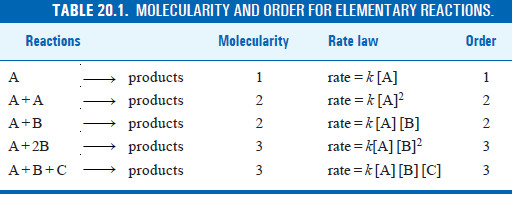
Molecularity of an Elementary Reaction
– The molecularity of an elementary reaction is defined as : the number of reactant molecules involved in a reaction.
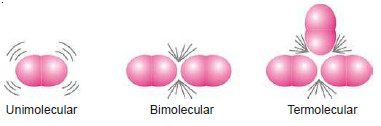
– Thus the molecularity of an elementary reaction is 1, 2, 3, etc., according as one, two or three reactant molecules are participating in the reaction.
– The elementary reactions having molecularity 1, 2 and 3 are called unimolecular, bimolecular and termolecular respectively.
– Thus we have :
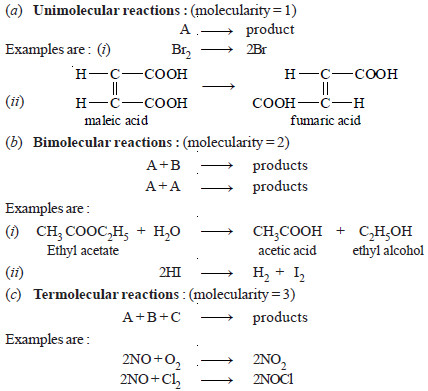
(a) Unimolecular reactions : (molecularity: 1)
(b) Bimolecular reactions : (molecularity = 2)
(c) Termolecular reactions : (molecularity = 3)
Why High Molecularity Reactions are Rare ?
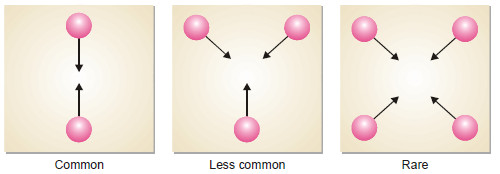
– Most of the reactions involve one, two or at the most three molecules.
– The reactions involving four or more molecules are very rare.
– The rarity of reactions with high molecularity can be explained on the basis of the kinetic molecular theory.
– According to this theory, the rate of a chemical reaction is proportional to the number of collisions taking place between the reacting molecules.
– The chances of simultaneous collision of reacting molecules will go on decreasing with increase in number of molecules.
– Thus the possibility of three molecules colliding together is much less than in case of bimolecular collision.
– For a reaction of molecularity 4, the four molecules must come closed and collide with one another at the same time.
– The possibility of their doing so is much less than even in the case of termolecular reaction.
– Hence the reactions involving many molecules proceed through a series of steps, each involving two or three or less number of molecules.
– Such a reaction is called a complex reaction and the slowest step determines the overall rate of the reactions.
Molecularity of a Complex Reaction
– Most chemical reactions are complex reactions.
– These occur in a series of steps. Each step is an elementary reaction.
– The stepwise sequence of elementary reactions that convert reactions to products is called the mechanism of the reaction.
– In any mechanism, some of the steps will be fast, others will be slow.
– A reaction can proceed no faster than its slowest step.
– Thus the slowest step is the ratedetermining step of the reaction.
– The decomposition of N2O5,
2N2O5 ⎯⎯→ 4NO2 + O2
is an example of a complex reaction. It occurs by the following steps :
– Each elementary reaction has its own molecularity equal to the number of molecules or atoms participating in it.
– It is meaningless to give the molecularity of the overall reaction because it is made of several elementary reactions, each, perhaps with a different molecularity.
– At best could be thought of as : the number of molecules or atoms taking part in the rate-determining step.
– Thus step 2 in the above mechanism is rate-determining and has molecularity ‘2’ which could be considered as the molecularity of the decomposition reaction of N2O5.
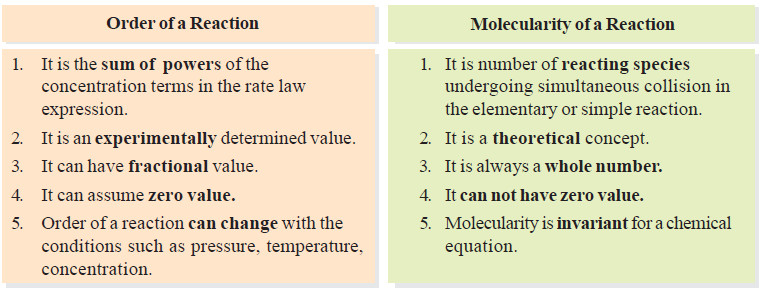
Molecularity versus order of reaction
– The term molecularity is often confused with order of a reaction.
– The total number of molecules or atoms which take part in a reaction as represented by the chemical equation, is known as the molecularity of reaction.
– The sum of the powers to which the concentrations are raised in the rate law is known as the order of reaction.
Molecularity and Order are Identical for Elementary Reactions or Steps
– The rate of an elementary reaction is proportional to the number of collisions between molecules (or atoms) of reactions.
– The number of collisions in turn is proportional to the concentration of each reactant molecule (or atom). Thus for a reaction.
– Two molecules of A and one molecule of B are participating in the reaction and, therefore, molecularity of the reaction is 2 + 1 = 3.
– The sum of powers in the rate law is 2 + 1 and hence the reaction order is also 3. Thus the molecularity and order for an elementary reaction are equal.
 Read Chemistry
Read Chemistry