Preparation of Sols and Purification of Sols
– In this topic, we will discuss Preparation of Sols, Purification of Sols and its colors
Preparation of Sols
– Lyophilic sols may be prepared by simply warming the solid with the liquid dispersion medium e.g., starch with water.
– On the other hand, lyophobic sols have to be prepared by special methods.
– These methods fall into two categories:
(a) Dispersion Methods
– in which larger macro-sized particles are broken down to colloidal size.
(b) Aggregation Methods
– in which colloidal size particles are built up by aggregating single ions or molecules.
Preparation of Sols by Dispersion methods
– In these methods, material in bulk is dispersed in another medium.
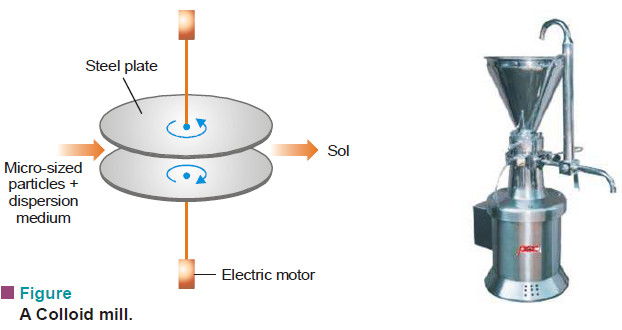
(1) Mechanical dispersion using Colloid mill
– The solid along with the liquid dispersion medium is fed into a Colloid mill.
– The mill consists of two steel plates nearly touching each other and rotating in opposite directions with high speed.
– The solid particles are ground down to colloidal size and are then dispersed in the liquid to give the sol.
– Colloidal graphite (a lubricant) and printing inks are made by this method.
– Recently, mercury sol has been prepared by disintegrating a layer of mercury into sol particles in water by means of ultrasonic vibrations.
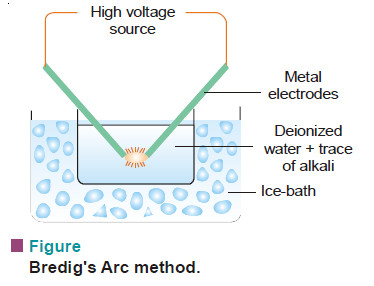
(2) Bredig’s Arc Method
– It is used for preparing hydrosols of metals e.g., silver, gold and platinum.
– An arc is struck between the two metal electrodes held close together beneath de-ionized water.
– The water is kept cold by immersing the container in ice/water bath and a trace of alkali (KOH) is added.
– The intense heat of the spark across the electrodes vaporises some of the metal and the vapour condenses under water.
– Thus the atoms of the metal present in the vapour aggregate to form colloidal particles in water.
– Since the metal has been ultimately converted into sol particles (via metal vapour), this method has been treated as of dispersion.
– Non-metal sols can be made by suspending coarse particles of the substance in the dispersion medium and striking an arc between iron electrodes.
(3) By Peptization
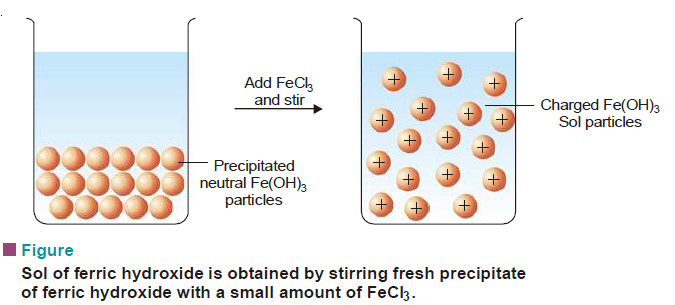
– Some freshly precipitated ionic solids are dispersed into colloidal solution in water by the addition of small quantities of electrolytes, particularly those containing a common ion.
– The precipitate adsorbs the common ions and electrically charged particles then split from the precipitate as colloidal particles.
– The dispersal of a precipitated material into colloidal solution by the action of an electrolyte in solution, is termed peptization.
– The electrolyte used is called a peptizing agent.
– Peptization is the reverse of coagulation of a sol.
Examples of preparation of sols by peptization
(1) Silver chloride, Ag+Cl–, can be converted into a sol by adding hydrochloric acid (Cl– being common ion.)
(2) Ferric hydroxide, Fe(OH)3, yields a sol by adding ferric chloride (Fe3+ being common ion).
Preparation of Sols by Aggregation Methods
– These methods consists of chemical reactions or change of solvent whereby the atoms or molecules of the dispersed phase appearing first, coalesce or aggregate to form colloidal particles.
– The conditions (temperature, concentration, etc.) used are such as permit the formation of sol particles but prevent the particles becoming too large and forming precipitate.
– The unwanted ions (spectator ions) present in the sol are removed by dialysis as these ions may eventually coagulate the sol.
– The more important methods for preparing hydrophobic sols are listed below:
(1) Double Decomposition
– An arsenic sulphide (As2S3) sol is prepared by passing a slow stream of hydrogen sulphide gas through a cold solution of arsenious oxide (As2O3).
– This is continued till the yellow colour of the sol attains maximum intensity.
As2O3 + 3H2S ⎯⎯→ As2S3 (sol) + 3H2O
– Excess hydrogen sulphide (electrolyte) is removed by passing in a stream of hydrogen.
(2) Reduction
– Silver sols and gold sols can be obtained by treating dilute solutions of silver nitrate or gold chloride with organic reducing agents like tannic acid or methanal (HCHO)
AgNO3 + tannic acid ⎯⎯→ Ag sol
AuCl3 + tannic acid ⎯⎯→ Au sol
(3) Oxidation
– A sol of sulphur is produced by passing hydrogen sulphide into a solution of sulphur dioxide.
2H2S + SO2 ⎯⎯→ 2H2O + S↓
– In qualitative analysis, sulphur sol is frequently encountered when H2S is passed through the solution to precipitate group 2 metals if an oxidizing agent (chromate or ferric ions) happen to be present.
– It can be removed by boiling (to coagulate the sulphur) and filtering through two filter papers folded together.
(4) Hydrolysis
– Sols of the hydroxides of iron, chromium and aluminium are readily prepared by the hydrolysis of salts of the respective metals.
– In order to obtain a red sol of ferric hydroxide, a few drops of 30% ferric chloride solution is added to a large volume of almost boiling water and stirred with a glass rod.
FeCl3 + 3H2O ⎯⎯→ Fe(OH)3 + 3HCl
(5) Change of Solvent
– When a solution of sulphur or resin in ethanol is added to an excess of water, the sulphur or resin sol is formed owing to decrease in solubility.
– The substance is present in molecular state in ethanol but on transference to water, the molecules precipitate out to form colloidal particles.
Purification of Sols
– In the methods of preparation stated above, the resulting sol frequently contains besides colloidal particles appreciable amounts of electrolytes.
– To obtain the pure sol, these electrolytes have to be removed.
– This purification of sols can be accomplished by three methods
- Dialysis
- Electrodialysis
- Ultrafiltration
(1) Dialysis
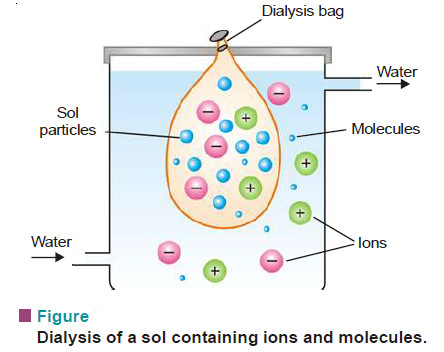
– Animal membranes (bladder) or those made of parchment paper and cellophane sheet, have very fine pores.
– These pores permit ions (or small molecules) to pass through but not the large colloidal particles.
– When a sol containing dissolved ions (electrolyte) or molecules is placed in a bag of permeable membrane dipping in pure water, the ions diffuse through the membrane.
– By using a continuous flow of fresh water, the concentration of the electrolyte outside the membrane tends to be zero.
– Thus diffusion of the ions into pure water remains brisk all the time.
– In this way, practically all the electrolyte present in the sol can be removed easily.
– The process of removing ions (or molecules) from a sol by diffusion through a permeable membrane is called Dialysis.
– The apparatus used for dialysis is called a Dialyser.
Example:
– A ferric hydroxide sol (red) made by the hydrolysis of ferric chloride will be mixed with some hydrochloric acid.
– If the impure sol is placed in the dialysis bag for some time, the outside water will give a white precipitate with silver nitrate.
– After a pretty long time, it will be found that almost the whole of hydrochloric acid has been removed and the pure red sol is left in the dialyser bag.
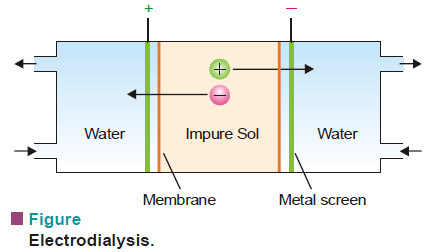
(2) Electrodialysis
– In this process, dialysis is carried under the influence of electric field.
– Potential is applied between the metal screens supporting the membranes.
– This speeds up the migration of ions to the opposite electrode. Hence dialysis is greatly accelerated.
– Evidently electrodialysis is not meant for nonelectrolyte impurities like sugar and urea.
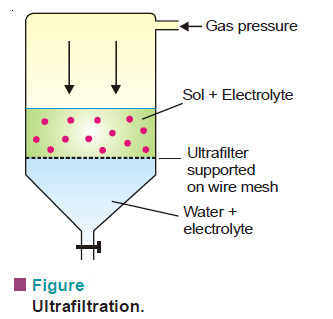
(3) Ultrafiltration
– Sols pass through an ordinary filter paper, Its pores are too large to retain the colloidal particles. However, if the filter paper is impregnated with collodion or a regenerated cellulose such as cellophane or visking, the pore size is much reduced. Such a modified filter paper is called an ultrafilter.
– The separation of the sol particles from the liquid medium and electrolytes by filtration through an ultrafilter is called ultrafiltration.
– Ultrafiltration is a slow process. Gas pressure (or suction) has to be applied to speed it up.
– The colloidal particles are left on the ultrafilter in the form of slime.
– The slime may be stirred into fresh medium to get back the pure sol.
– By using graded ultrafilters, the technique of ultrafiltration can be employed to separate sol particles of different sizes.
The colour of sols
– The colour of a hydrophobic sol depends on the wavelength of the light scattered by the dispersed particles.
– The wavelength of the scattered light again depends on the size and the nature of the particles.
– This is fully borne out from the following date in case of silver sols.