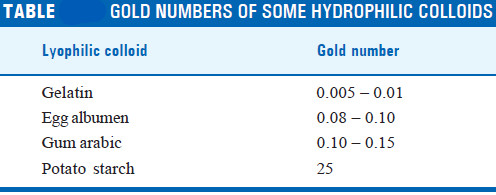Electrical Properties of Sols
Electrical Properties of Sols
– In this topic we will discuss the Electrical Properties of Sols as follow:
(1) The sol particles carry an electric charge.
(2) Electrophoresis.
(3) Electro-osmosis.
(4) Coagulation or Precipitation.
(5) Protective action of sols.
(6) Origin of charge on sol particles.
(1) The sol particles carry an electric charge
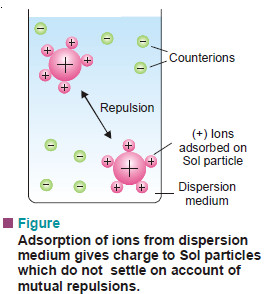
– The most important property of colloidal dispersions is that all the suspended particles posses either a positive or a negative charge.
– The mutual forces of repulsion between similarly charged particles prevent them from aggregating and settling under the action of gravity. This gives stability to the sol.
– The sol particles acquire positive or negative charge by preferential adsorption of positive or negative ions from the dispersion medium.
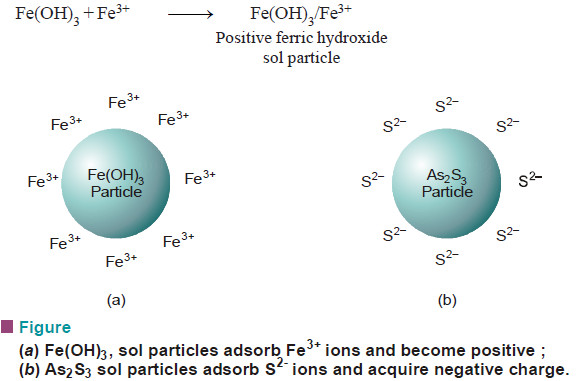
– For example, a ferric hydroxide sol particles are positively charged because these adsorb Fe3+ ions from ferric chloride (FeCl3) used in the preparation of the sol.
– Since the sol as a whole is neutral, the charge on the particle is counterbalanced by oppositely charged ions termed counterions (in this case Cl–) furnished by the electrolyte in medium.
Electrical Double layer
– The surface of colloidal particle acquires a positive charge by selective adsorption of a layer of positive ions around it.
– This layer attracts counterions from the medium which form a second layer of negative charges.
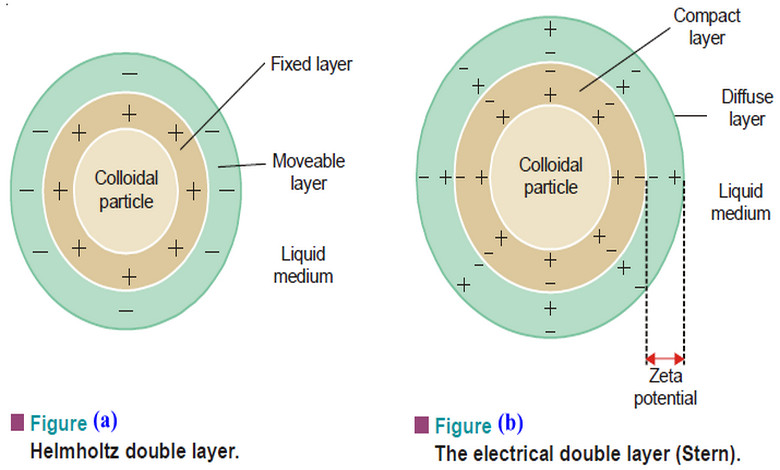
The combination of the two layer of +ve and –ve charges around the sol particle was called Helmholtz Double layer.
– Helmholtz thought that positive charges next to the particle surface were fixed, while the layer of negative charges along with the medium were mobile.
– More recent considerations have shown that the double layer is made of :
(a) a Compact layer of positive and negative charges which are fixed firmly on the particle surface.
(b) a Diffuse layer of counterions (negative ions) diffused into the medium containing positive ions.
– The combination of the compact and diffuse layer is referred to as the Stern Double layer after the colloid chemist who first realised its significance.
– The diffuse layer is only loosely attached to the particle surface and moves in the opposite direction under an applied electric field.
– Because of the distribution of the charge around the particle, there is a difference in potential between the compact layer and the bulk of solution across the diffuse layer. This is called by Electrokinetic or Zeta potential.
– The presence of the double layer accounts for the electrical properties : (a) Cataphoresis; and (b) Electro-osmosis of colloids.
– It has been made possible to estimate the magnitude of the zeta potential with the help of these properties.
– We have explained the theory of electrical double layer taking example of a positive sol.
– Our considerations could well be applied to a negative sol with the interchange of the disposition of positive and negative ions.
(2) Electrophoresis
– If electric potential is applied across two platinum electrodes dipping in a hydrophilic sol, the dispersed particles move toward one or the other electrode.
– The movement of sol particles under an applied electric potential is called electrophoresis or cataphoresis.
– If the sol particles migrate toward the positive electrode, they carry a negative charge.
– On the other hand, if they move toward the negative electrode, they are positively charged.
– Thus by noting the direction of movement of the sol particles, we can determine whether they carry a positive or negative charge.
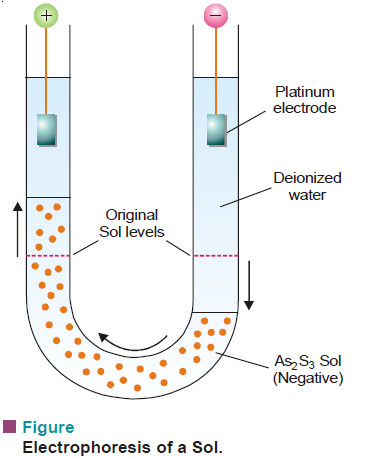
– The phenomenon of electrophoresis can be demonstrated by placing a layer of As2S3 sol under two limbs of a U-tube.
– When a potential difference of about 100 volts is applied across the two platinum electrodes dipping in deionised water, it is observed that the level of the sol drops on the negative electrode side and rises on the positive electrode side.
– This shows that As2S3 sol has migrated to the positive electrode, indicating that the particles are negatively charged.
– Similarly, a sol of ferric hydroxide will move to the negative electrode, showing that its particles carry positive charge.
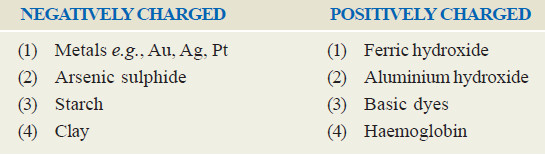
– Using water as the dispersion medium, the charge on the particles of some common sols determined by electrophoresis is given below.
Applications of electrophoresis
– Some important applications of electrophoresis are :
(1) Removal of smoke from chimney gases;
(2) Removal of suspended impurities;
(3) Electro-plating of rubber on metal surfaces from latex (a sol);
(4) painting of metal parts of cars from colloidal pigments.
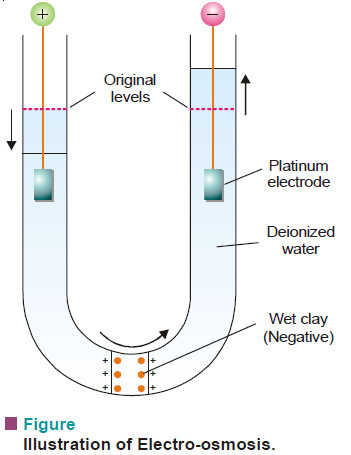
(3) Electro-osmosis
– A sol is electrically neutral. Therefore the dispersion medium carries an equal but opposite charge to that of the dispersed particles.
– Thus the medium will move in opposite direction to the dispersed phase under the influence of applied electric potential.
– When the dispersed phase is kept, stationary, the medium is actually found to move to the electrode of opposite sign that its own.
– The movement of the dispersion medium under the influence of applied potential is known as electroosmosis.
– Electro-osmosis is a direct consequence of the existence of zeta potential between the sol particles and the medium.
– When the applied pressure exceeds the zeta potential, that diffuse layer moves and causes electro-osmosis.
– The phenomenon of electro-osmosis can be demonstrated by using a U-tube in which a plug of wet clay (a negative colloid) is fixed (see Fig above).
– The two limbs of the tube are filled with water to the same level.
– The platinum electrodes are immersed in water and potential applied across them. It will be observed that water level rises on the cathode side and falls on anode side.
– This movement of the medium towards the negative electrode, shows that the charge on the medium is positive.
– Similarly, for a positively charged colloid electro-osmosis will take place in the reverse direction.
– Technically the phenomenon has been applied in the removal of water from peat, in dewatering of moist clay and in drying dye pastes.
(4) Coagulation or Precipitation
– We know that the stability of a lyophobic sol is due to the adsorption of positive or negative ions by the dispersed particles.
– The repulsive forces between the charged particles do not allow them to settle.
– If, some how, the charge is removed, there is nothing to keep the particles apart from each other.
– They aggregate (or flocculate) and settle down under the action of gravity.
– The flocculation and settling down of the discharged sol particles is called coagulation or precipitation of the sol.
How coagulation can be brought about?
– The coagulation or precipitation of a given sol can be brought about in four ways :
(a) By addition of electrolytes
(b) By electrophoresis
(c) By mixing two oppositely charged sols
(d) By boiling
(a) By addition of Electrolytes.
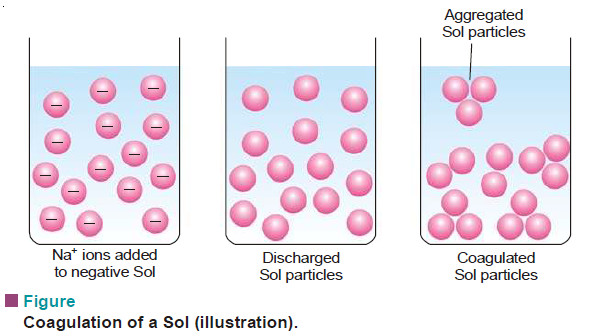
– When excess of an electrolyte is added to a sol, the dispersed particles are precipitated.
– The electrolyte furnishes both positive and negative ions in the medium.
– The sol particles adsorb the oppositely charged ions and get discharged.
– The electrically neutral particles then aggregate and settle down as precipitate.
– A negative ion (anion) causes the precipitation of a positively charged sol, and vice versa.
– The effectiveness of an anion or cation to precipitate a sol, will naturally depend on the magnitude of the charge or valence of the effective ion.
– From a study of the precipitating action of various electrolytes on particular sol, Hardy and Schulze gave a general rule.
– Hardy-Schulze Rule states that the precipitating effect of an ion on dispersed phase of opposite charge increases with the valence of the ion.
– The higher the valency of the effective ion, the greater is its precipitating power.
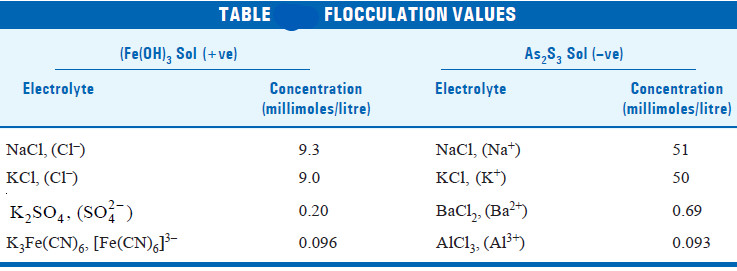
– Thus for precipitating an As2S3 sol (negative), the precipitating power of Al3+ , Ba2+, Na+ ions is in the order
Al3+ > Ba2+ > Na+
– Similarly, for precipitating Fe(OH)3 sol (positive), the precipitating power of cations [Fe(CN)6]3–, SO4 , Cl– is in the order.
[Fe(CN)6]3– > SO42– > Cl–
– The precipitation power of an electrolyte or ion is experimentally determined by finding the minimum concentration in millimoles per litre required to cause the precipitation of a sol in 2 hours. This is called the Flocculation value.
– The smaller the flocculation value the higher the precipitating power of an ion.
– It may be noted how rapidly the precipitation power increases with the increases of valence.
– The ratio for the mono-, di-, and trivalent anion or cation are approximately 1 : 40 : 90 for Fe(OH)3 sol and 1 : 70 : 500 for the As2S3 sol.
(b) By Electrophoresis.
– In electrophoresis the charged sol particles migrate to the electrode of opposite sign.
– As they come in contact with the electrode, the particles are discharged and precipitated.
(c) By mixing two oppositely charged sols.
– The mutual coagulation of two sols of opposite charge can be effected by mixing them.
– The positive particles of one sol are attracted by the negative particles of the second sol.
– This is followed by mutual adsorption and precipitation of both the sols.
– Ferric hydroxide (+ve sol) and arsenious sulphide (–ve sol) form such a pair.
(d) By boiling.
– Sols such as sulphur and silver halides dispersed in water, may be coagulated by boiling.
– Increased collisions between the sol particles and water molecules remove the adsorbed electrolyte.
– This takes away the charge from the particles which settle down.
(5) Protective action of sols
– Lyophobic sols are readily precipitated by small amounts of electrolytes. However these sols are often stabilized by the addition of lyophilic sols.
– The property of lyophilic sols to prevent the precipitation of a lyophobic sol is called protection.
– The lyophilic sol used to protect a lyophobic sol from precipitation is referred to as a Protective colloid.
Example
– If a little gelatin (hydrophilic colloid) is added to a gold sol (hydrophobic sol), the latter is protected.
– The ‘protected gold sol’ is no longer precipitated on the addition of sodium chloride.
Explanation
– The particles of the hydrophobic sol adsorb the particles of the lyophilic sol. Thus the lyophilic colloid forms a coating around the lyophobic sol particles.
– The hydrophobic colloid, therefore, behaves as a hydrophilic sol and is precipitated less easily by electrolytes.
Gold number
– The lyophilic colloids differ widely in their powers of protection.
– The protective action of different colloids is measured in terms of the ‘Gold number’ introduced by Zsigmondy.
– The gold number is defined as :the number of milligrams of a hydrophilic colloid that will just prevent the precipitation of 10 ml of a gold sol on the addition of 1 ml of 10 per cent sodium chloride solution.
– The onset of precipitation of the gold sol is indicated by a colour change from red to blue when
the particle size just increases.
– The gold numbers of hydrophilic colloids are given in the following Table.
– The smaller the gold number of a hydrophilic colloid, the greater is its protective power.
– Gelatin has a small gold number and is an effective protective colloid.
– Starch has a very high value, which shows that it is an ineffective protective colloid.
– The use of protective colloids to stabilize colloidal systems is widespread.
– In the preparation of ice cream, gelatin is added to act as a protecting agent to the colloidal particles of ice.
– If the ice particles coagulate, the smooth texture of ice cream is lost.
– Argyrol, used in eye drops, is a sol of silver protected by organic material.
(6) Origin of charge on sol particles
– All the dispersed particles of a particular sol carry a positive or a negative charge.
– They acquire this charge by
(a) Adsorption of ions from the aqueous medium
(b) Ionisation of surface groups
(a) By Adsorption of ions
– In most cases the charge on the sol particles originates by the selective adsorption of ions common to the particles from the dispersion medium.
Examples.
(i) Ferric hydroxide sol particles are positive because they adsorb the common ion Fe3+ from the aqueous medium.
(ii) Arsenic sulphide sol particles acquire a negative charge since they adsorb the common ion S2– from the medium.
– It is not necessary that a particular sol particles always adsorb the same kind of ions.
– In fact, the particles may adsorb the anions or cations whichever are in excess and acquire the corresponding charge.
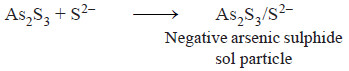
– For example, AgCl sol produced by the addition of AgNO3 solution to sodium chloride solution, bears a positive charge if Ag+ ions are in excess.
– On the other hand, if Cl– ions are in excess, the AgCl sol particles acquire a negative charge.
(b) Ionization of Surface groups
(1) Charge on Soaps and Detergent sols.
– Soaps and detergent sol particles are aggregates of many molecules.
– The hydrocarbons tails of the molecules are directed to the centre, while the groups – COO–Na+ (or – OS – O3 Na+ ) constitute the surface in contact with water.
– As a result of ionization of the surface groups, the particle surface is now made of the anionic heads – COO– (or – OSO3– ). This makes the sol particle negative.
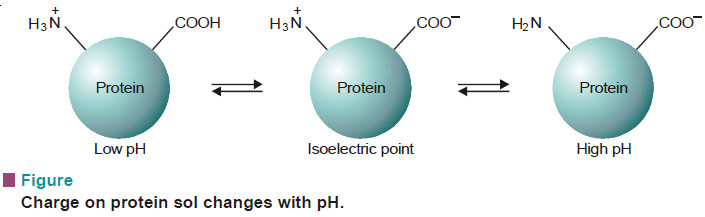
(2) Charge on Protein sols.
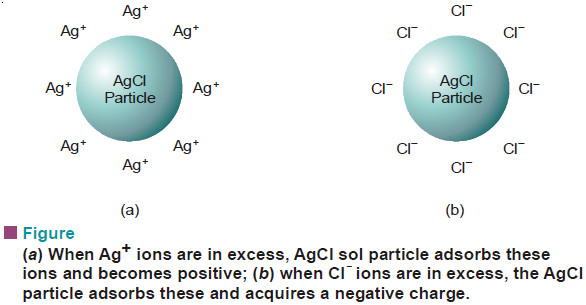
– Protein sol particles possess both acidic and basic functional groups.
– In aqueous solution at low pH, the –NH2 group (basic) acquires a proton to give –NH3+ while at high pH the —COOH group (acidic) transfers a proton to OH– to give —COO–.
– Thus the protein sol particle has positive charge at low pH and negative charge at high pH.
– At an intermediate pH called the isoelectric point, the particles will be electrically neutral.
– The changes in the charge of the protein sol are shown by the direction of movement of the dispersed phase in electrophoresis.