Synthesis of Epoxides
– In this topic, we will talk about Synthesis of Epoxides by two methods: Peroxyacid epoxidation and Base-promoted cyclization of halohydrins.
Synthesis of Epoxides
– Epoxides are easily made from alkenes, and (unlike other ethers) they undergo a variety of useful synthetic reactions.
– For these reasons, epoxides are valuable synthetic intermediates.
– Here we review the epoxidation techniques already covered and consider in more detail the useful syntheses and reactions of epoxides.
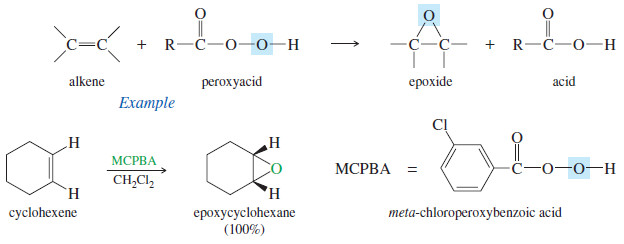
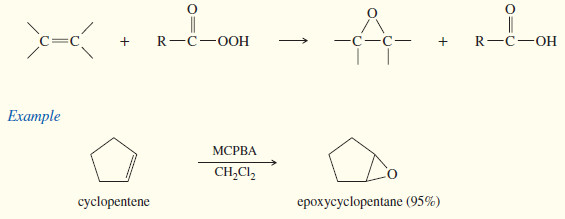
(1) Peroxyacid Epoxidation
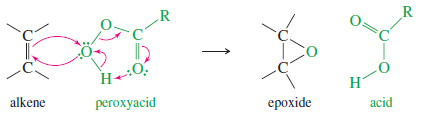
– Peroxyacids (sometimes called peracids) are used to convert alkenes to epoxides.
– If the reaction takes place in aqueous acid, the epoxide opens to a glycol. Therefore, to make an epoxide, we avoid strong acids.
– Because of its desirable solubility properties, metachloroperoxybenzoic acid (MCPBA) is often used for these epoxidations.
– MCPBA is a weakly acidic peroxyacid that is soluble in aprotic solvents such as CH2Cl2.
– The epoxidation takes place in a one-step, concerted reaction that maintains the stereochemistry of any substituents on the double bond.
– The peroxyacid epoxidation is quite general, with electron-rich double bonds reacting fastest.
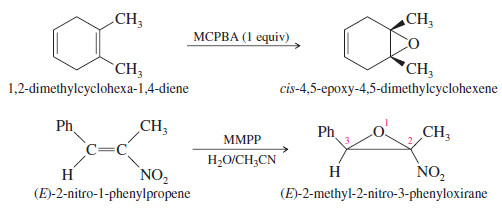
– The following reactions are difficult transformations made possible by this selective, stereospecific epoxidation procedure.
– The second example uses magnesium monoperoxyphthalate (MMPP), a relatively stable water-soluble peroxyacid often used in large-scale epoxidations.
– These aqueous MMPP epoxidations, carried out at neutral pH to avoid opening the epoxide, avoid the large-scale use of hazardous chlorinated solvents.
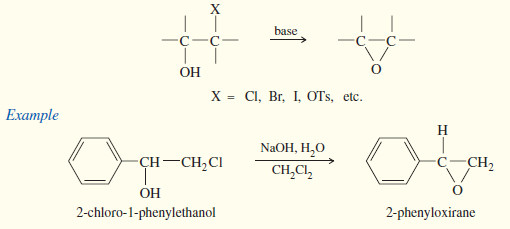
(2) Base-Promoted Cyclization of Halohydrins
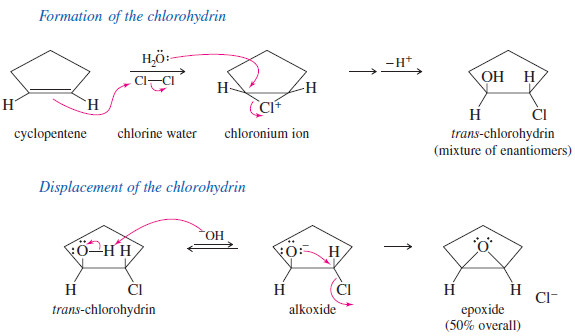
– A second synthesis of epoxides and other cyclic ethers involves a variation of the Williamson ether synthesis.
– If an alkoxide ion and a halogen atom are located in the same molecule, the alkoxide may displace a halide ion and form a ring.
– Treatment of a halohydrin with base leads to an epoxide through this internal SN2 attack.
– Halohydrins are easily generated by treating alkenes with aqueous solutions of halogens.
– Bromine water and chlorine water add across double bonds with Markovnikov orientation.
– The following reaction shows cyclopentene reacting with chlorine water to give the chlorohydrin.
– Treatment of the chlorohydrin with aqueous sodium hydroxide gives the epoxide.
– This reaction can be used to synthesize cyclic ethers with larger rings.
– The difficulty lies in preventing the base (added to deprotonate the alcohol) from attacking and displacing the halide.
– 2,6-Lutidine, a bulky base that cannot easily attack a carbon atom, can deprotonate the hydroxyl group to give a five-membered cyclic ether.
– Five-, six-, and seven-membered (and occasionally four-membered) cyclic ethers are formed this way.
Summary of Synthesis of Epoxides
1. Peroxyacid epoxidation
2. Base-promoted cyclization of halohydrins
References:
- Organic chemistry / L.G. Wade, Jr / 8th ed, 2013 / Pearson Education, Inc. USA.
- Fundamental of Organic Chemistry / John McMurry, Cornell University/ 8th ed, 2016 / Cengage Learningm, Inc. USA.
- Organic Chemistry / T.W. Graham Solomons, Craig B. Fryhle , Scott A. Snyder / 11 ed, 2014/ John Wiley & Sons, Inc. USA.
- Unergraduate Organic Chemistry /Dr. Jagdamba Singh, Dr. L.D.S Yadav / 1st ed, 2010/ Pragati prakashan Educational Publishers, India.