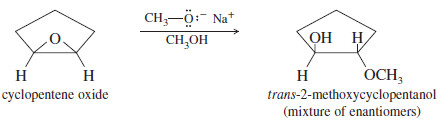Ring Opening of Epoxides
– In this topic, we will talk aboutAcid-Catalyzed Ring Opening of Epoxides, Base-Catalyzed Ring Opening of Epoxides, and Orientation of Epoxide Ring Opening.
Acid-Catalyzed Ring Opening of Epoxides
– Epoxides are much more reactive than common dialkyl ethers because of the large strain energy (about 105 kJ/mol or 25 kcal/mol) associated with the three-membered ring.
– Unlike other ethers, epoxides react under both acidic and basic conditions.
– The products of acid-catalyzed opening depend primarily on the solvent used.
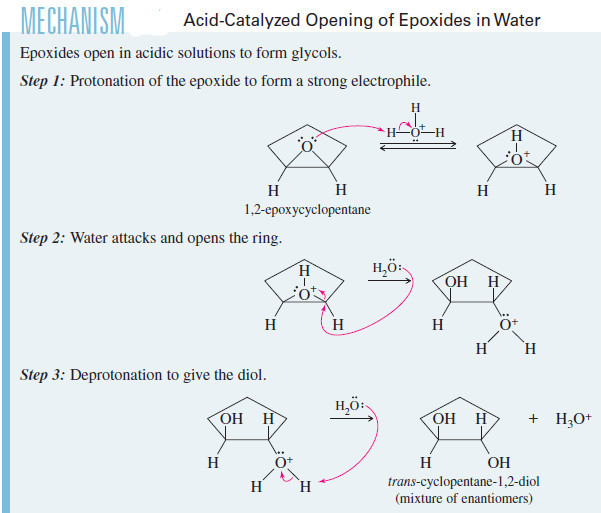
In Water
– we saw that acid-catalyzed hydrolysis of epoxides gives glycols with anti stereochemistry.
– The mechanism of this hydrolysis involves protonation of oxygen (forming a good leaving group), followed by SN2 attack by water.
– Anti stereochemistry results from the back side attack of water on the protonated epoxide.
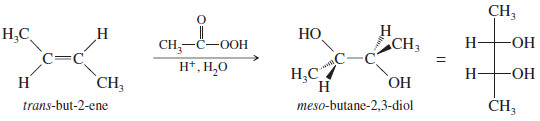
– Direct anti hydroxylation of an alkene (without isolation of the epoxide intermediate) is possible by using an acidic aqueous solution of a peroxyacid.
– As soon as the epoxide is formed, it hydrolyzes to the glycol.
– Peroxyacetic acid (CH3CO3H) and peroxyformic acid (HCO3H) are often used for the anti hydroxylation of alkenes.
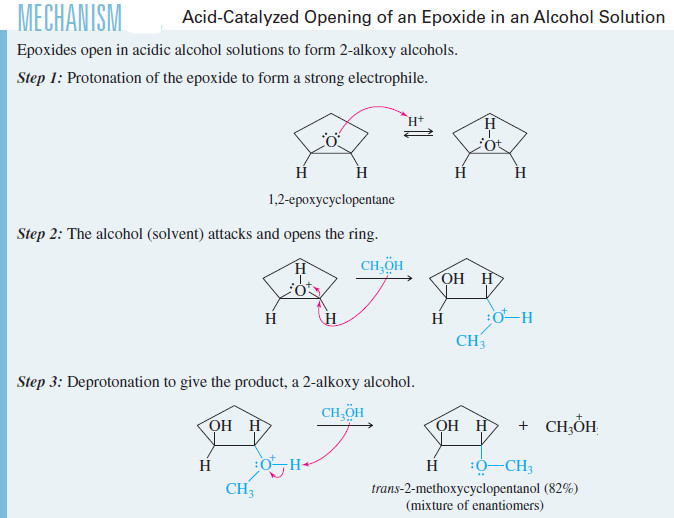
In Alcohols
– When the acid-catalyzed opening of an epoxide takes place with an alcohol as the solvent, a molecule of alcohol acts as the nucleophile. This reaction produces an alkoxy alcohol with anti stereochemistry.
– This is an excellent method for making compounds with ether and alcohol functional groups on adjacent carbon atoms.
– For example, the acid-catalyzed opening of 1,2 epoxycyclopentane in a methanol solution gives trans-2-methoxycyclopentanol.
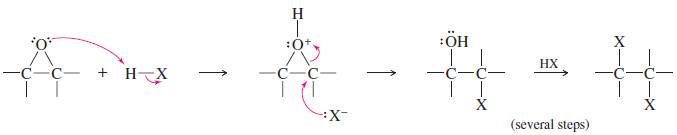
Using Hydrohalic Acids
– When an epoxide reacts with a hydrohalic acid (HCl, HBr, or HI), a halide ion attacks the protonated epoxide.
– This reaction is analogous to the cleavage of ethers by HBr or HI.
– The halohydrin initially formed reacts further with HX to give a 1,2-dihalide.
– This is rarely a useful synthetic reaction, because the 1,2-dihalide can be made directly from the alkene by electrophilic addition of X2.
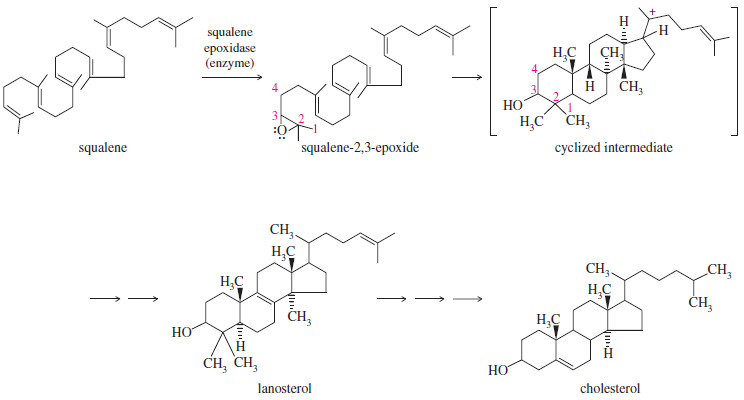
The Opening of Squalene-2,3-Epoxide
– Steroids are tetracyclic compounds that serve a wide variety of biological functions, including hormones (sex hormones), emulsifiers (bile acids), and membrane components (cholesterol).
– The biosynthesis of steroids is believed to involve an acid-catalyzed opening of squalene-2,3 epoxide.
– Squalene is a member of the class of natural products called terpenes.
– The enzyme squalene epoxidase oxidizes squalene to the epoxide,
– which opens and forms a carbocation that cyclizes under the control of another enzyme.
– The cyclized intermediate rearranges to lanosterol, which is converted to cholesterol and other steroids.
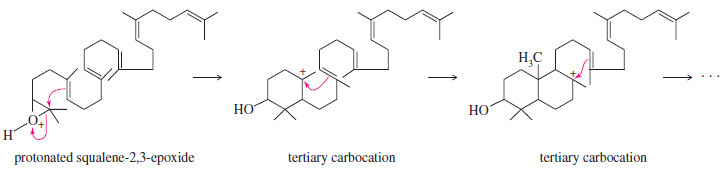
– Although cyclization of squalene-2,3-epoxide is controlled by an enzyme, its mechanism is similar to the acid-catalyzed opening of other epoxides.
– The epoxide oxygen becomes protonated and is attacked by a nucleophile.
– In this case, the nucleophile is a pi bond. The initial result is a tertiary carbocation.
– This initial carbocation is attacked by another double bond, leading to the formation of another ring and another tertiary carbocation.
– A repetition of this process leads to the cyclized intermediate shown in Figure above.
– Note that this sequence of steps converts an achiral, acyclic starting material (squalene) into a compound with four rings and seven asymmetric carbon atoms.
– The enzyme-catalyzed sequence takes place with high yields and complete stereospecificity, providing a striking example of asymmetric induction in a biological system.
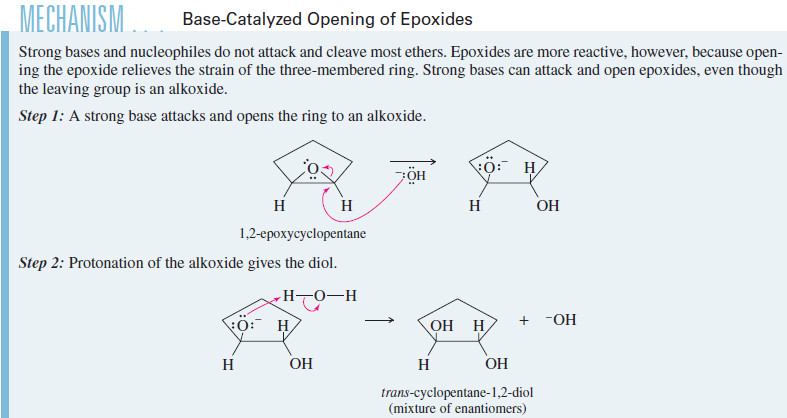
Base-Catalyzed Ring Opening of Epoxides
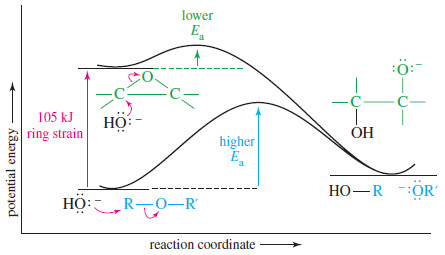
– Most ethers do not undergo nucleophilic substitutions or eliminations under basic conditions, because an alkoxide ion is a poor leaving group.
– Epoxides have about 105 kJ mol (25 kcal mol) of ring strain that is released upon ring opening, however, and this strain is enough to compensate for the poor alkoxide leaving group.
– The following Figure compares the energy profiles for nucleophilic attack on an ether and on an epoxide.
– The starting epoxide is about 105 kJ mol (25 kcal mol) higher in energy than the ether, and its displacement has a lower activation energy.
– The reaction of an epoxide with hydroxide ion leads to the same product as the acid-catalyzed opening of the epoxide: a 1,2-diol (glycol), with anti stereochemistry.
– In fact, either the acid catalyzed or base-catalyzed reaction may be used to open an epoxide, but the acid-catalyzed reaction takes place under milder conditions.
– Unless there is an acid-sensitive functional group present, the acid-catalyzed hydrolysis is preferred.
– Like hydroxide, alkoxide ions react with epoxides to form ring-opened products.
– For example, cyclopentene oxide reacts with sodium methoxide in methanol to give the same trans-2-methoxycyclopentanol produced in the acid-catalyzed opening in methanol.
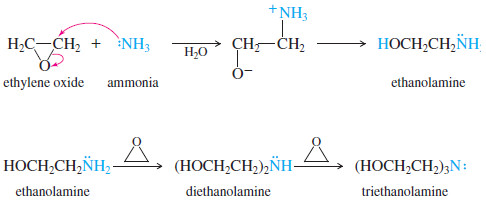
– Amines can also open epoxides.
– Ethylene oxide reacts with aqueous ammonia to
give ethanolamine, an important industrial reagent.
– The nitrogen atom in ethanolamine is still nucleophilic, and ethanolamine can react further to give diethanolamine and triethanolamine.
– Good yields of ethanolamine are achieved by using excess ammonia.
Orientation of Epoxide Ring Opening
– Symmetrically substituted epoxides (such as cyclopentene oxide, above) give the same product
in both the acid-catalyzed and base-catalyzed ring openings.
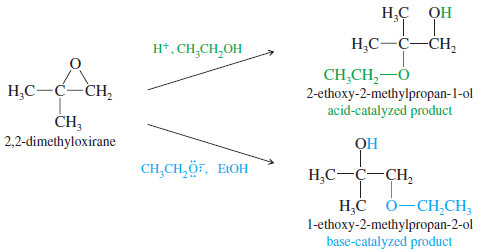
– An unsymmetrical epoxide may produce different products under acid-catalyzed and base- catalyzed conditions, however.
Under basic conditions
– Under basic conditions, the alkoxide ion simply attacks the less hindered carbon
atom in an SN2 displacement.
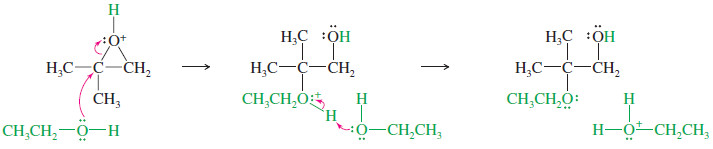
Under acidic conditions
– Under acidic conditions, the alcohol attacks the protonated epoxide.
– It might seem that the alcohol would attack at the less hindered oxirane carbon, but this is not the case.
– In the protonated epoxide, there is a balancing act between ring strain and the energy it costs to put some of the positive charge on the carbon atoms.
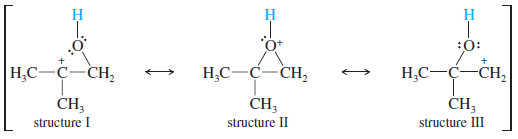
– We can represent this sharing of positive charge by drawing resonance forms that suggest what the cations would look like if the ring started to open.
– These “no-bond” resonance forms help us to visualize the charge distribution in the protonated epoxide.
– Structure II is the conventional structure for the protonated epoxide, while structures I and III show that the oxirane carbon atoms share part of the positive charge.
– The tertiary carbon bears a larger part of the positive charge, and it is more strongly electrophilic; that is, structure I is more important than structure III.
– The bond between the tertiary carbon and oxygen is weaker, implying a lower transition state energy for attack at the tertiary carbon.
– Attack by the weak nucleophile (ethanol in this case) is sensitive to the strength of the electrophile, and it occurs at the more electrophilic tertiary carbon.
– This ring opening is similar to the opening of a bromonium ion in the formation of a bromohydrin and the opening of the mercurinium ion during oxymercuration .
– All three reactions involve the opening of an electrophilic threemembered ring by a weak nucleophile.
– Attack takes place at the more electrophilic carbon atom, which is usually the more substituted carbon because it can better support the positive charge.
– Most base-catalyzed epoxide openings, on the other hand, involve attack by a strong nucleophile at the less hindered carbon atom.
References:
- Organic chemistry / L.G. Wade, Jr / 8th ed, 2013 / Pearson Education, Inc. USA.
- Fundamental of Organic Chemistry / John McMurry, Cornell University/ 8th ed, 2016 / Cengage Learningm, Inc. USA.
- Organic Chemistry / T.W. Graham Solomons, Craig B. Fryhle , Scott A. Snyder / 11 ed, 2014/ John Wiley & Sons, Inc. USA.
- Unergraduate Organic Chemistry /Dr. Jagdamba Singh, Dr. L.D.S Yadav / 1st ed, 2010/ Pragati prakashan Educational Publishers, India.