Writing Equations for Organic Reactions
– In this subject, we will discuss Writing Equations for Organic Reactions.
Writing Equations for Organic Reactions
(1) Like other reactions, equations for organic reactions are usually drawn with a single reaction arrow (→) between the starting material and product, but other conventions make these equations look different from those encountered in general chemistry.
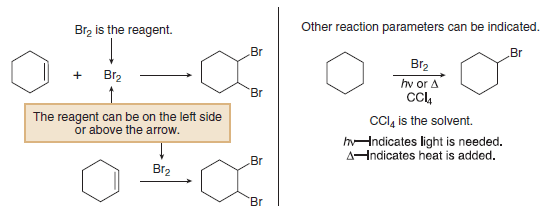
(2) The reagent, the chemical substance with which an organic compound reacts, is sometimes drawn on the left side of the equation with the other reactants.
– At other times, the reagent is drawn above the reaction arrow itself, to focus attention on the organic starting material by itself on the left side.
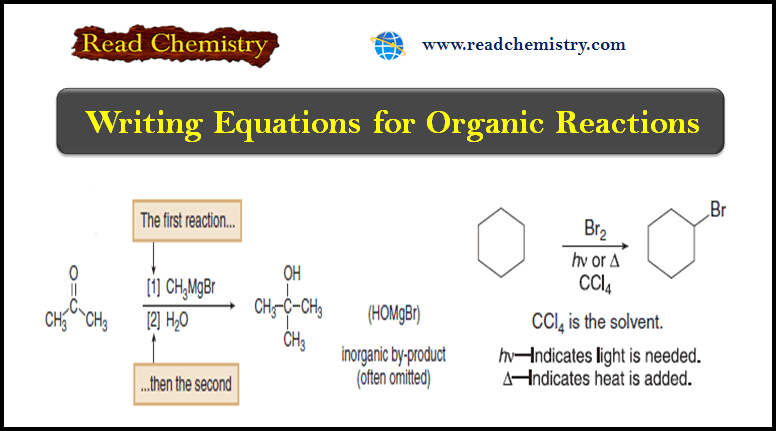
(3) The solvent and temperature of a reaction may be added above or below the arrow.
(4) The symbols (hʋ) and (Δ) are used for reactions that require light or heat, respectively.
– The Following figure presents an organic reaction in different ways.
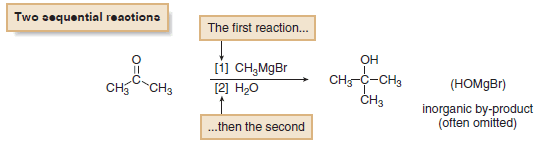
(5) When two sequential reactions are carried out without drawing any intermediate compound, the steps are usually numbered above or below the reaction arrow.
– This convention signifies that the first step occurs before the second, and the reagents are added in sequence, not at the same time.
– In this equation, only the organic product is drawn on the right side of the arrow.
– Although the reagent CH3MgBr contains both Mg and Br, these elements do not appear in the organic product, and they are often omitted on the product side of the equation.
– These elements have not disappeared.
– They are part of an inorganic by-product (HOMgBr in this case) and are often of little interest to an organic chemist.
Notes during Writing Equations for Organic Reactions
(1) Often the solvent and temperature of a reaction are omitted from chemical equations, to further focus attention on the main substances involved in the reaction.
(2) Most organic reactions take place in a liquid solvent.
– Solvents solubilize key reaction components and serve as heat reservoirs to maintain a given temperature.
Reference: Organic chemistry / Janice Gorzynski Smith, University of Hawai’i at Manoa / (Third edition), 2011. USA