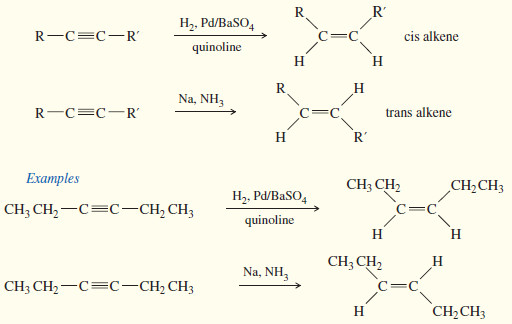
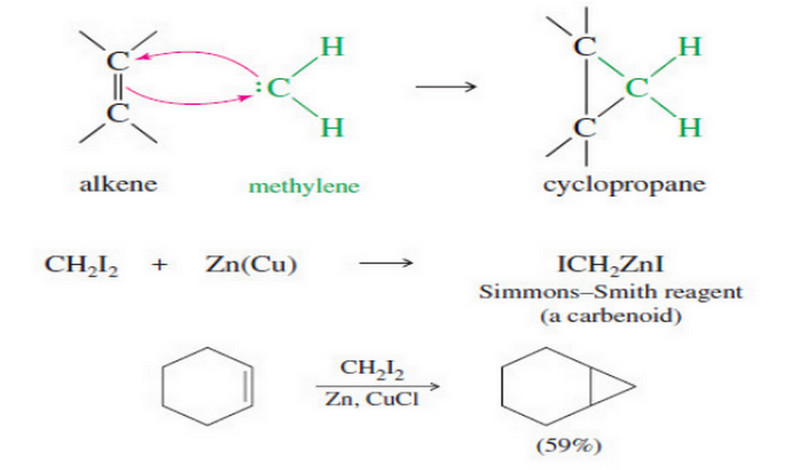
The Molecular Orbitals of Benzene
In this subject, we will talk about The Molecular Orbitals of Benzene
The Molecular Orbitals of Benzene
– Visualizing benzene as a resonance hybrid of two Kekulé structures cannot fully explain the unusual stability of the aromatic ring.
– As we have seen with other conjugated systems, molecular orbital theory provides the key to understanding aromaticity and predicting which compounds will have the stability of an aromatic system.
– Benzene has a planar ring of six sp2 hybrid carbon atoms, each with an unhybridized p orbital that overlaps with the p orbitals of its neighbors to form a continuous ring of orbitals above and below the plane of the carbon atoms.
– Six pi electrons are contained in this ring of overlapping p orbitals.
– The six overlapping p orbitals create a cyclic system of molecular orbitals.
– Cyclic systems of molecular orbitals differ from linear systems such as buta-1,3-diene and the allyl system.
– A two dimensional cyclic system requires two-dimensional MOs, with the possibility of two distinct MOs having the same energy.
Molecular Orbital Representation for benzene
– We can still follow the same principles in developing a molecular orbital representation for benzene, however.
(1) There are six atomic p orbitals that overlap to form the benzene pi system. Therefore, there must be six molecular orbitals.
(2) The lowest-energy molecular orbital is entirely bonding, with constructive overlap between all pairs of adjacent p orbitals. There are no vertical nodes in this lowest-lying MO.
(3) The number of nodes increases as the MOs increase in energy.
(4) The MOs should be evenly divided between bonding and antibonding MOs, with the possibility of nonbonding MOs in some cases.
(5) We expect that a stable system will have filled bonding MOs and empty antibonding MOs.
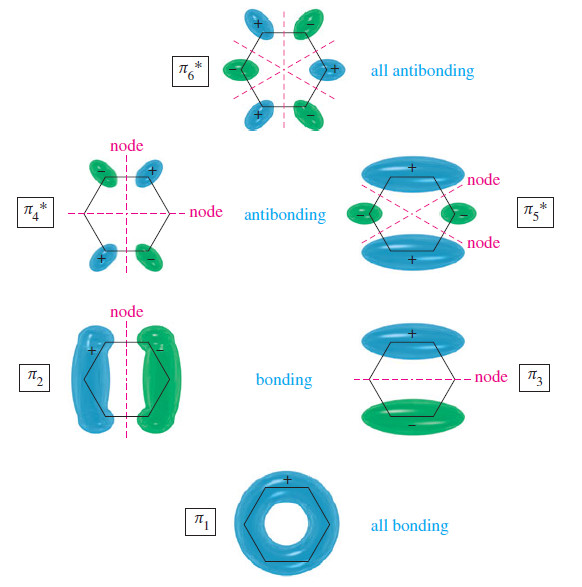
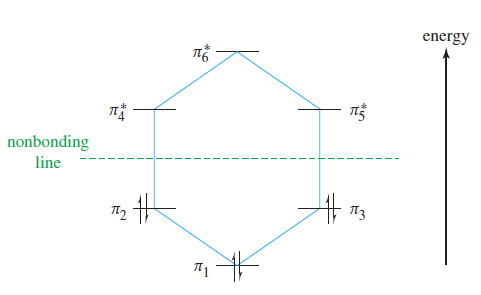
– The following Figure shows the six 𝜋 molecular orbitals of benzene as viewed from above,
showing the sign of the top lobe of each p orbital.
– The first MO (𝜋1) is entirely bonding, with no nodes. It is very low in energy because it has six bonding interactions and the electrons are delocalized over all six carbon atoms.
– The top lobes of the p orbitals all have the same sign, as do the bottom lobes.
– The six p orbitals overlap to form a continuously bonding ring of electron density.
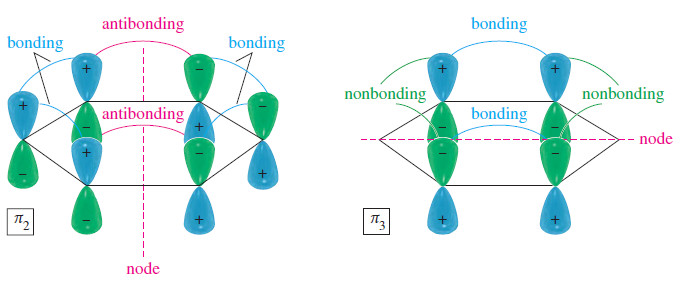
– In a cyclic system of overlapping p orbitals, the intermediate energy levels are degenerate (equal in energy), with two orbitals at each energy level.
– Both 𝜋2 and 𝜋3 one nodal plane, as we expect at the second energy level.
– Notice that 𝜋2 has four bonding interactions and two antibonding interactions, for a total of two net bonding interactions.
– Similarly, 𝜋3 has two bonding interactions and four nonbonding interactions, also totaling two net bonding interactions.
– Although we cannot use the number of bonding and antibonding interactions as a quantitative measure of an orbital’s energy, it is clear that 𝜋2 and 𝜋3 are bonding MOs, but not as strongly bonding as 𝜋1.
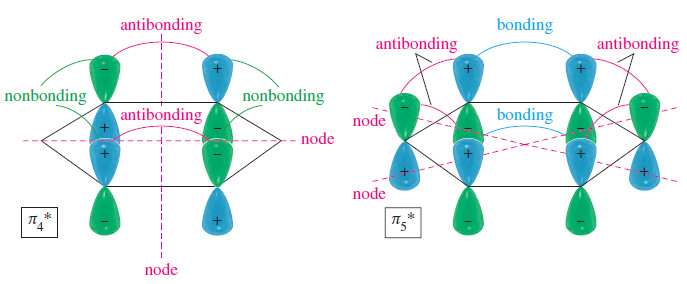
– The next orbitals, 𝜋4* and 𝜋5* are also degenerate, with two nodal planes in each.
– The 𝜋4* orbital has two antibonding interactions and four nonbonding interactions; it is an antibonding (*) orbital.
– Its degenerate partner,𝜋5*, has four antibonding interactions and two bonding interactions, for a total of two antibonding interactions.
– This degenerate pair of MOs, 𝜋4* and 𝜋5* are about as strongly antibonding as 𝜋2 and 𝜋3 are bonding.
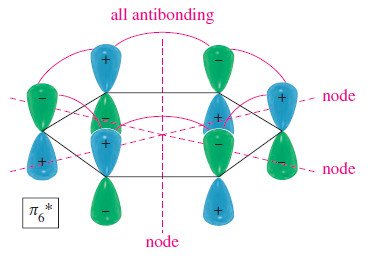
– The all-antibonding 𝜋6* has three nodal planes.
– Each pair of adjacent p orbitals is out of phase and interacts destructively.
The Energy Diagram of Benzene
– The energy diagram of the benzene MOs (see Figure) shows them to be symmetrically distributed above and below the nonbonding line (the energy of an isolated p orbital).
– The all-bonding and all antibonding orbitals (𝜋4*and 𝜋6* ) are lowest and highest in energy, respectively.
– The degenerate bonding orbitals 𝜋2 and 𝜋3 are higher in energy than 𝜋1 but still bonding.
– The degenerate pair 𝜋4* and 𝜋6* are anti-bonding, yet not as high in energy as the all-antibonding 𝜋6* orbital.
– The Kekulé structure for benzene shows three pi bonds, representing six electrons (three pairs) involved in pi bonding.
– Six electrons fill the three bonding MOs of the benzene system.
– This electronic configuration explains the unusual stability of benzene.
– The first MO is all-bonding and is particularly stable.
– The second and third (degenerate) MOs are still strongly bonding, and all three of these bonding MOs delocalize the electrons over several nuclei.
– This configuration, with all the bonding MOs filled (a “closed bonding shell”), is energetically very favorable.
References:
- Organic chemistry / L.G. Wade, Jr / 8th ed, 2013 / Pearson Education, Inc. USA.
- Fundamental of Organic Chemistry / John McMurry, Cornell University/ 8th ed, 2016 / Cengage Learningm, Inc. USA.
- Organic Chemistry / T.W. Graham Solomons, Craig B. Fryhle , Scott A. Snyder / 11 ed, 2014/ John Wiley & Sons, Inc. USA.
- Unergraduate Organic Chemistry /Dr. Jagdamba Singh, Dr. L.D.S Yadav / 1st ed, 2010/ Pragati prakashan Educational Publishers, India.