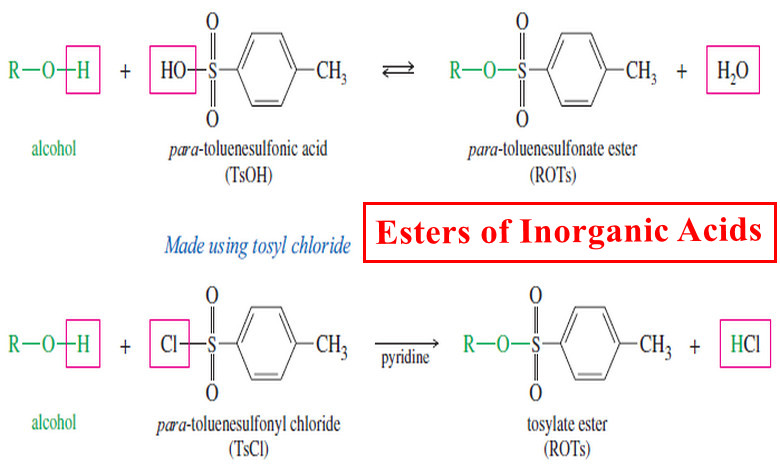
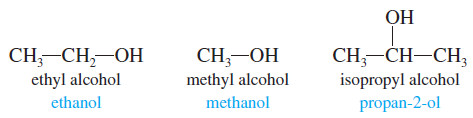Esterification of Alcohols
– In this subject we will talk about the Esterification of Alcohols.
What are Alcohols?
– Alcohols are organic compounds containing hydroxyl (-OH) groups.
– They are some of the most common and useful compounds in nature, in industry, and around the house.
– The word alcohol is one of the oldest chemical terms, derived from the early Arabic al-kuhl.
– Originally it meant (the powder) and later (the essence). Ethyl alcohol, distilled from wine, was considered to be (the essence) of wine.
– Ethyl alcohol (grain alcohol) is found in alcoholic beverages, cosmetics, and drug preparations. Methyl alcohol (wood alcohol) is used as a fuel and solvent.
– Isopropyl alcohol (rubbing alcohol) is used as a skin cleanser for injections and minor cuts.
– Alcohols are synthesized by a wide variety of methods, and the hydroxyl group may be converted to most other functional groups.
– For these reasons, alcohols are versatile synthetic intermediates.
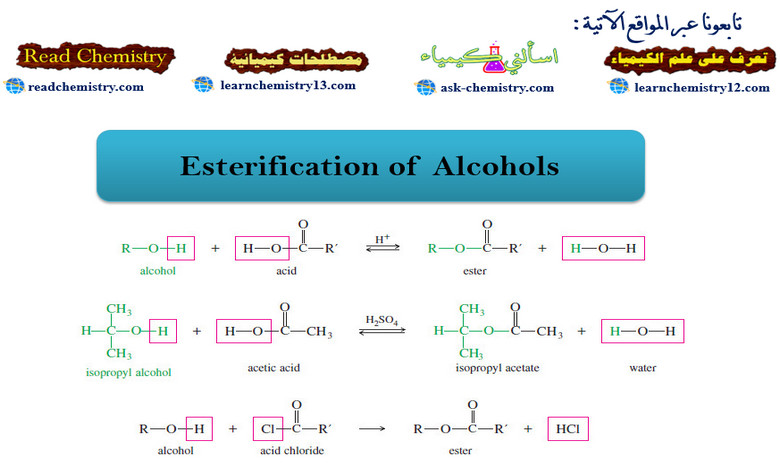
Esterification of Alcohols
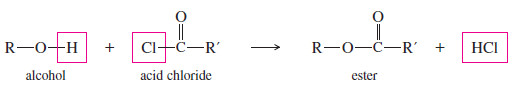
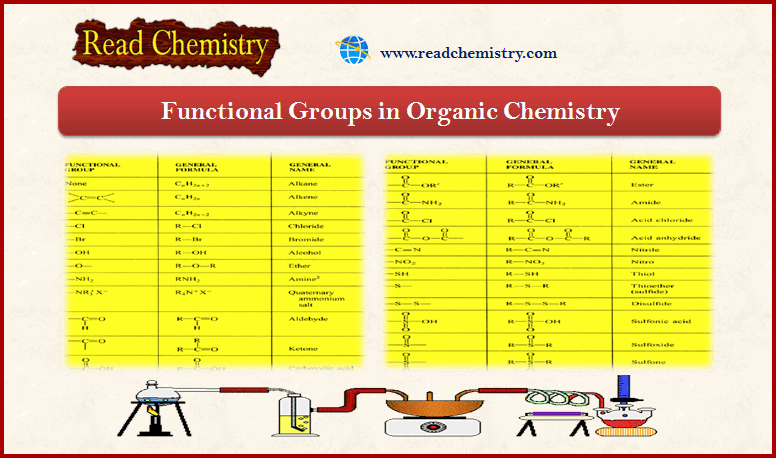
– To an organic chemist, the term ester normally means an ester of a carboxylic acid, unless some other kind of ester is specified.
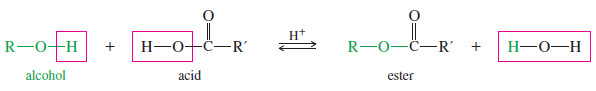
– Replacing the group (-OH) of a carboxylic acid with the (-OR) group of an alcohol gives a carboxylic ester.
– The following condensation, called the Fischer esterification, shows the relationship between the alcohol and the acid on the left and the ester and water on the right.
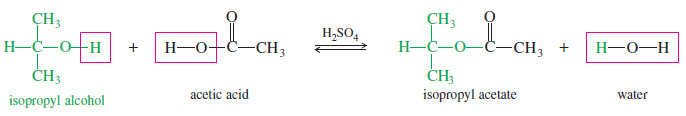
– For example, if we mix isopropyl alcohol with acetic acid and add a drop of sulfuric acid as a catalyst, the following equilibrium results.
– Because the Fischer esterification is an equilibrium (often with an unfavorable equilibrium constant), clever techniques are often required to achieve good yields of esters.
– For example, we can use a large excess of the alcohol or the acid. Adding a dehydrating agent removes water (one of the products), driving the reaction to the right.
– There is a more powerful way to form an ester, however, without having to deal with an unfavorable equilibrium.
– An alcohol reacts with an acid chloride in an exothermic reaction to give an ester.