Number of Signals in NMR Spectroscopy
The Number of Signals
– In general, the number of NMR signals corresponds to the number of different kinds of protons present in the molecule.
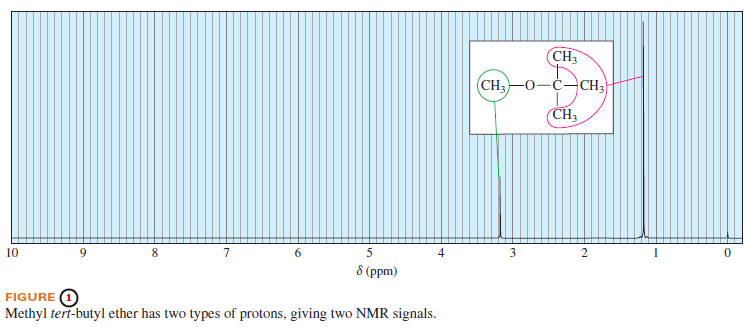
– For example, methyl tert-butyl ether has two types of protons (Figure 1).
– The three methoxy protons are chemically identical, and they give rise to a single absorption at δ 3.2.
– The tert-butyl protons are chemically different from the methoxy protons, absorbing at δ 1.2.
– Protons in identical chemical environments with the same shielding have the same chemical shift. Such protons are said to be chemically equivalent.
– This is what is meant whenever we use the term equivalent in discussing NMR spectroscopy.
– In methyl tertbutyl ether, the three methoxy protons are chemically equivalent, and the nine tert butyl protons are chemically equivalent.
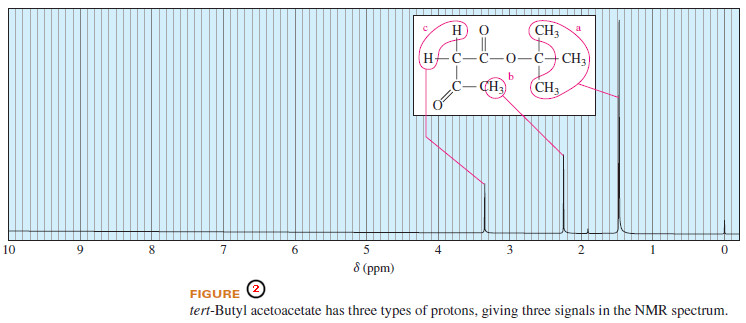
– The spectrum of tert-butyl acetoacetate (Figure 2) shows three types of protons: the tert-butyl protons (a), with a chemical shift of δ 1.5, the methyl protons (b), deshielded by an adjacent carbonyl group, with a chemical shift of δ 2.25 and the methylene protons (c), deshielded by two adjacent carbonyl groups, at δ 3.35.
– In some cases, there may be fewer signals in the NMR spectrum than there are different types of protons in the molecule.
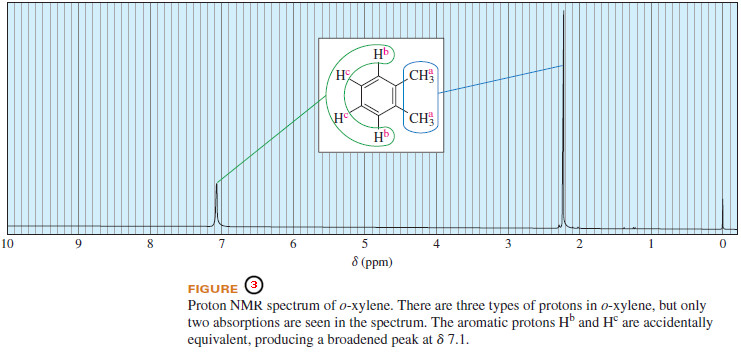
– For example, Figure (3) shows the structure and spectrum of o-xylene (1,2-dimethylbenzene).
– There are three different types of protons, labeled a for the two equivalent methyl groups, b for the protons adjacent to the methyl groups, and c for the protons two carbons removed.
– The spectrum shows only two distinct signals, however. The upfield signal at δ 3.2 corresponds to the six methyl protons, Ha .
– The absorption at δ 7.2 corresponds to all four of the aromatic protons, Hb and Hc .
– Although the two types of aromatic protons are different, the methyl groups do not strongly influence the electron density of the ring or the amount of shielding felt by any of the substituents on the ring.
– The aromatic protons produce two signals, but these signals happen to occur at nearly the same chemical shift.
– Protons that are not chemically equivalent but happen to absorb at the same chemical shift are said to be accidentally equivalent.