General Chemistry
General Chemistry introduces basic chemical concepts, including atomic structure, bonding, reactions, stoichiometry, states of matter, and periodic trends. It lays the groundwork for advanced study in all chemistry branches.
-
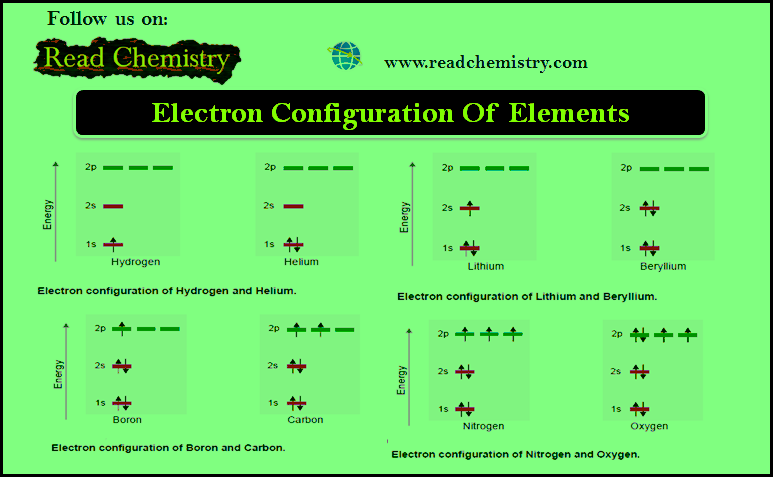
Electron Configuration Of Elements
– In this subject, we will discuss the rules of Electron Configuration Of Elements Electron Configuration Of Elements – We…
Read More » -
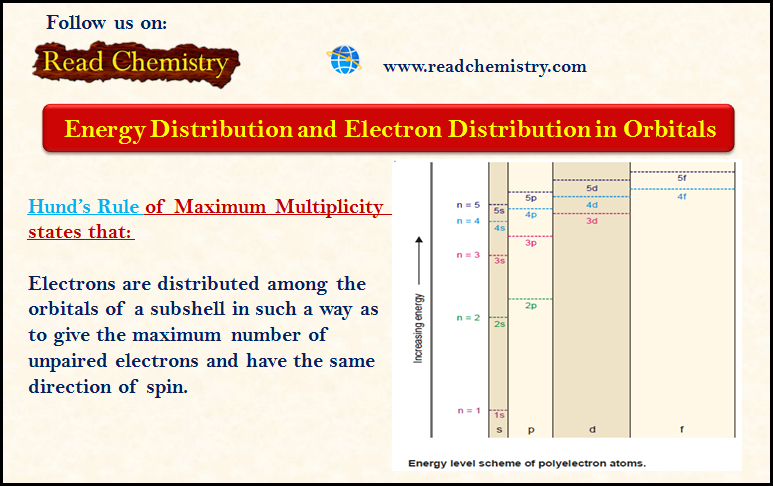
Distribution of Electrons in Orbitals
– In this subject, we will discuss the Distribution of Electrons in Orbitals according to Hund’s Rule. Energy Distribution and…
Read More » -
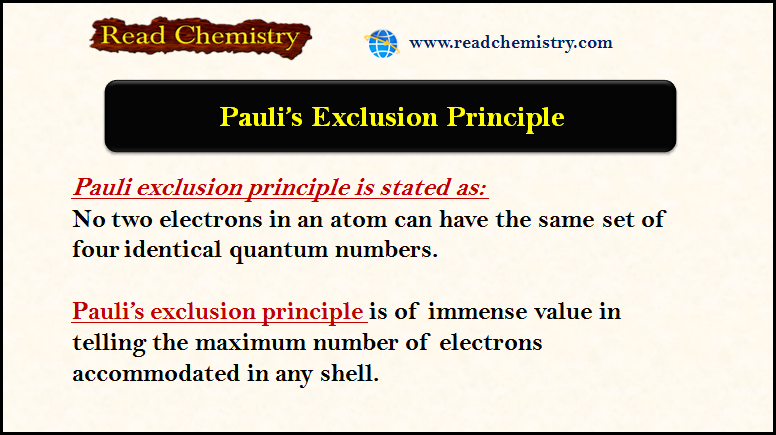
Pauli Exclusion Principle
– The Pauli exclusion principle is of immense value in telling the maximum number of electrons accommodated in any shell.…
Read More » -
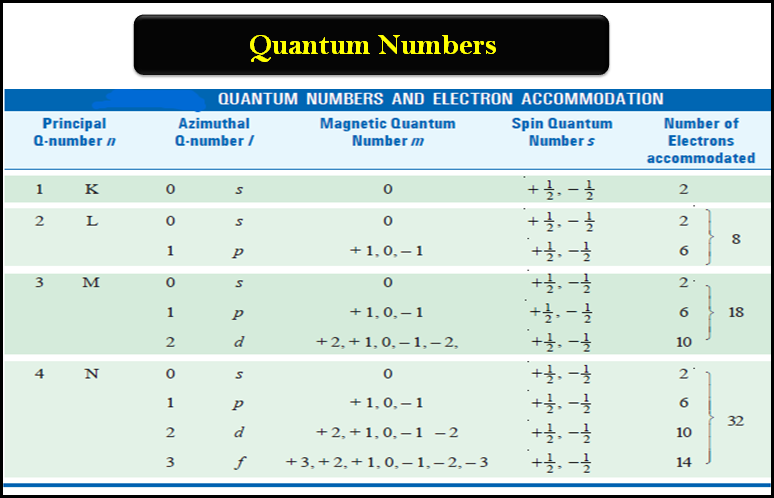
Quantum Numbers (Principal, Azimuthal, Magnetic and Spin)
Quantum Numbers – Bohr’s electronic energy shells or levels, designated as Principal Quantum Numbers (n), could hardly explain the hydrogen…
Read More » -
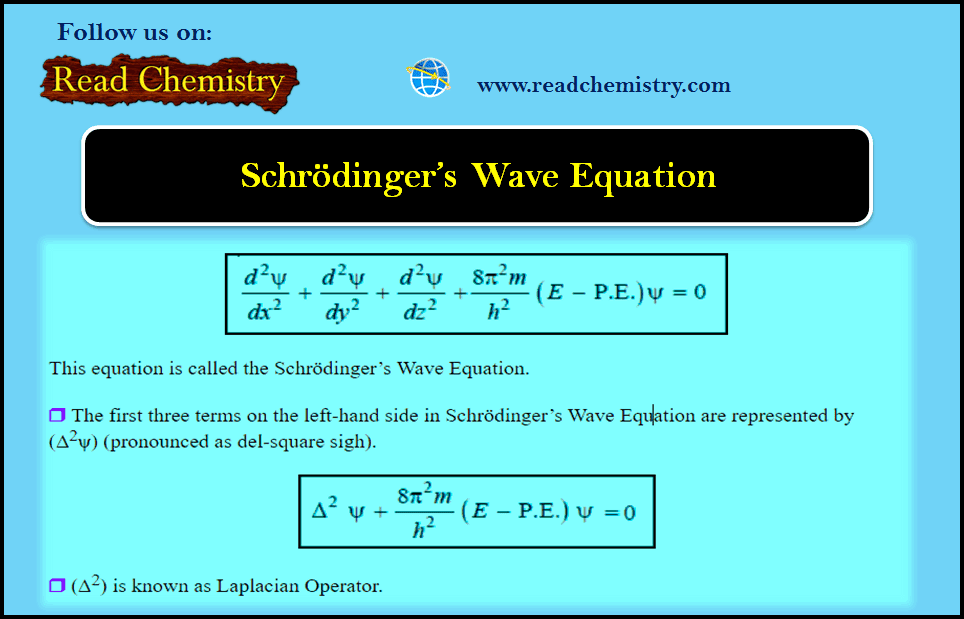
Schrödinger Wave Equation
Schrödinger Wave Equation – In order to provide sense and meaning to the probability approach, Schrödinger derived an equation known…
Read More » -
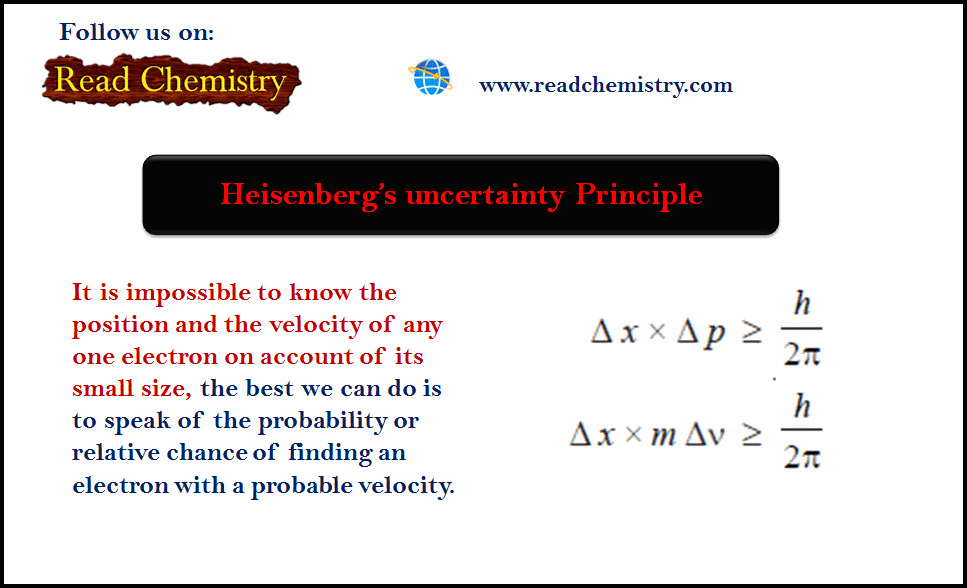
Heisenberg’s uncertainty Principle
– Heisenberg’s uncertainty Principle: As it is impossible to know the position and the velocity of any one electron on…
Read More » -
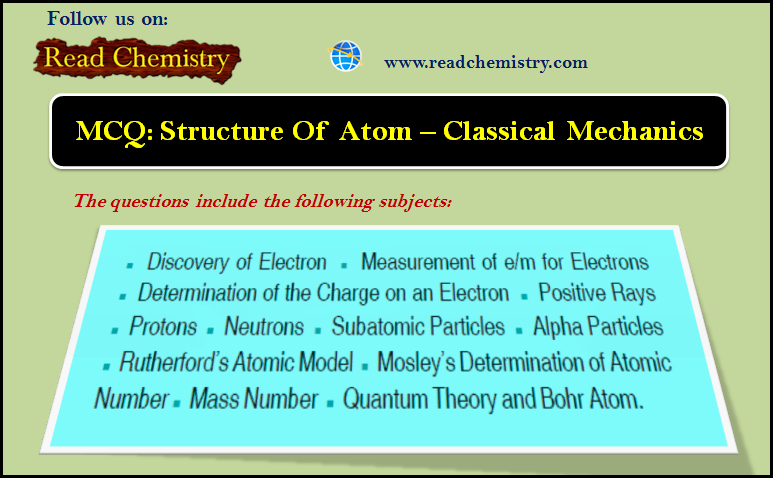
MCQ: Structure of atom – Classical Mechanics
1. In the spectrum of hydrogen atom, the series which falls in ultraviolet region is_______ (a) Lyman series (b) Balmer…
Read More » -
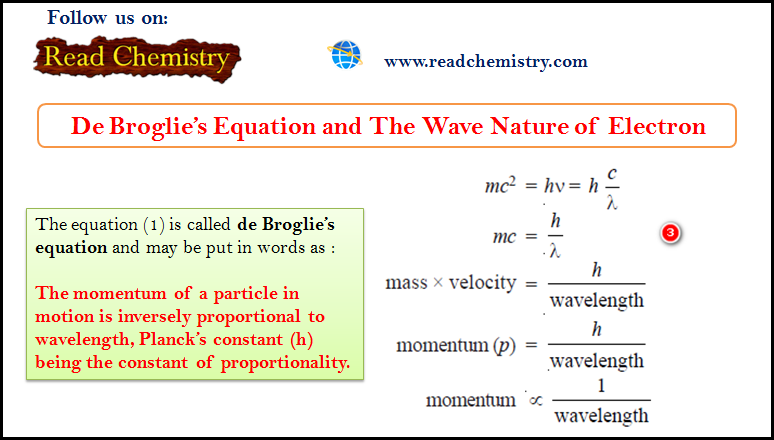
De Broglie Equation – The Wave Nature of Electron
De Broglie Equation – de Broglie had arrived at his hypothesis (de Broglie equation) with the help of Planck’s Quantum…
Read More » -
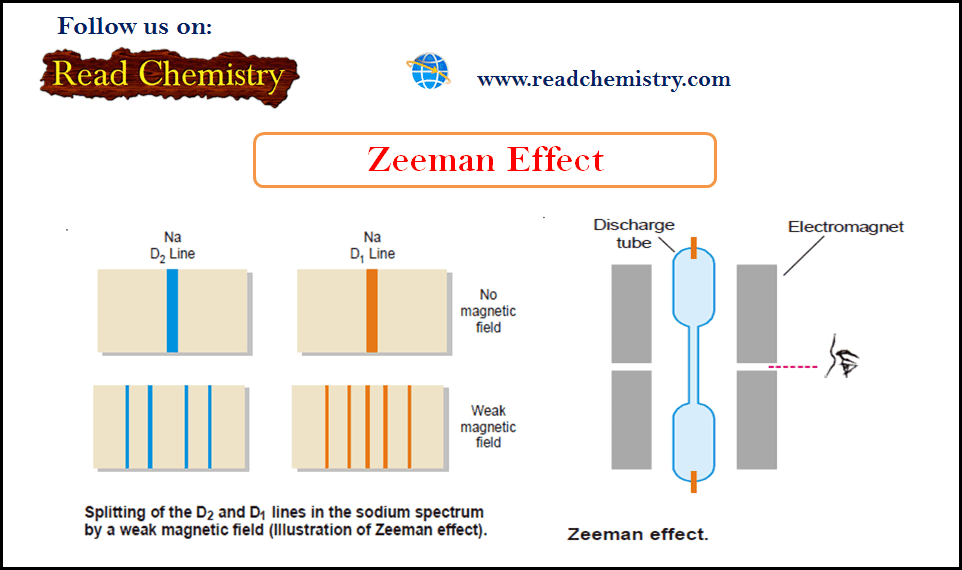
Zeeman Effect
Zeeman Effect – In 1896 Zeeman discovered that spectral lines are split up into components when the source emitting lines…
Read More » -
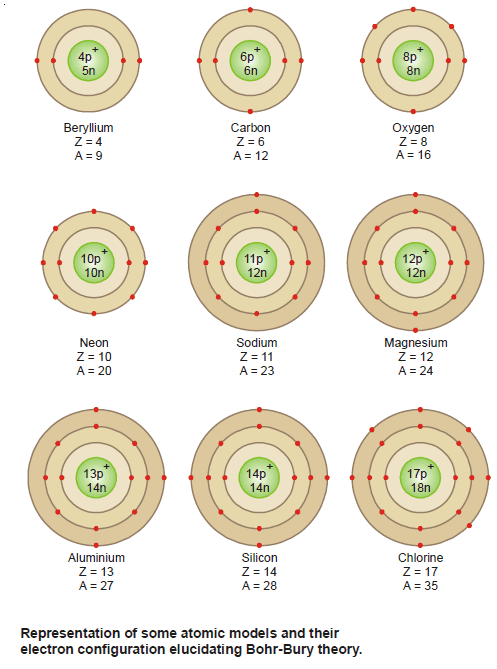
Bohr-Bury Scheme – Electron arrangement in the orbits
– The Bohr-Bury scheme considers that the maximum number of electrons that each orbit can contain is 2 × n2,…
Read More » -
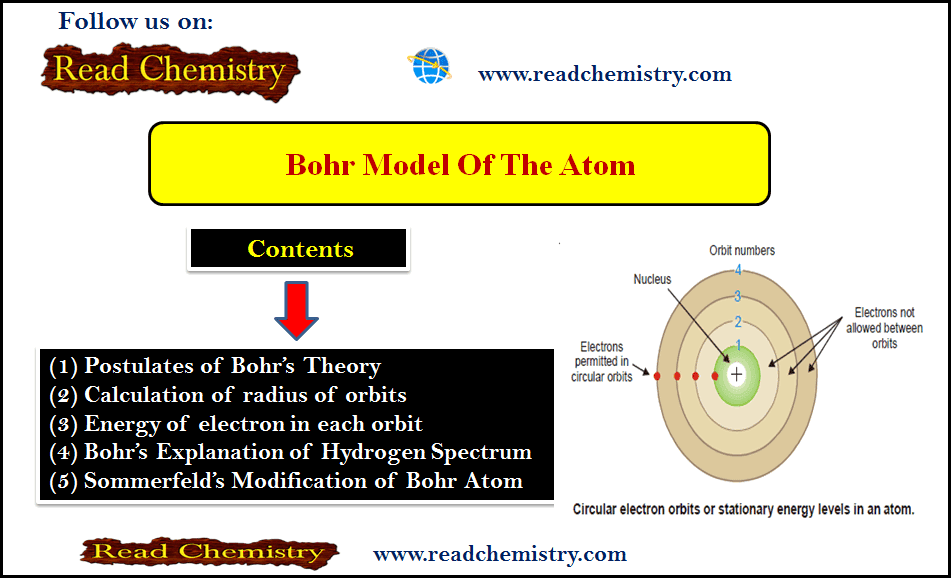
Bohr Model of atom – Bohr Theory
– Bohr theory was based on Planck’s quantum theory and was built on some postulates. – Rutherford’s nuclear model simply…
Read More » -
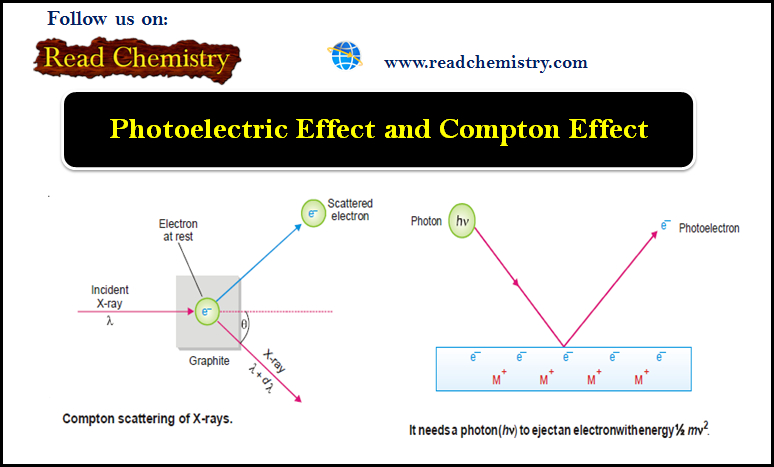
Photoelectric Effect and Compton Effect
– In this subject, we will discuss the Photoelectric Effect and Compton Effect Photoelectric Effect – When a beam of…
Read More » -
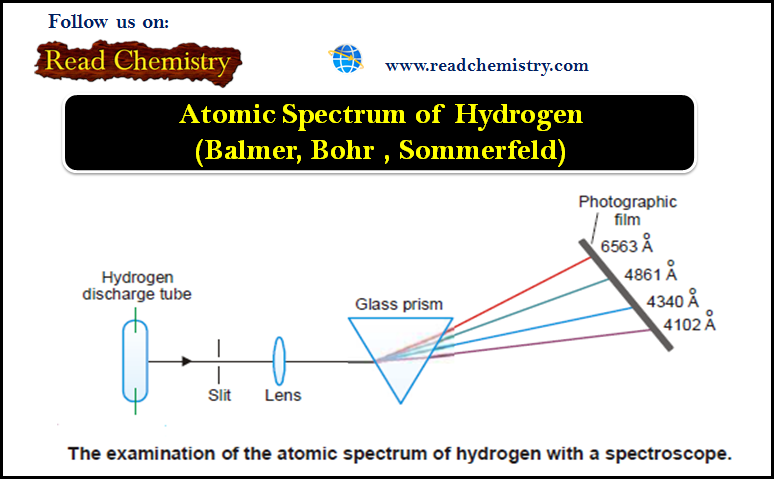
Atomic Spectrum of Hydrogen
– In this subject, we will discuss the atomic Spectrum of Hydrogen Atomic Spectrum – When an element in the…
Read More » -
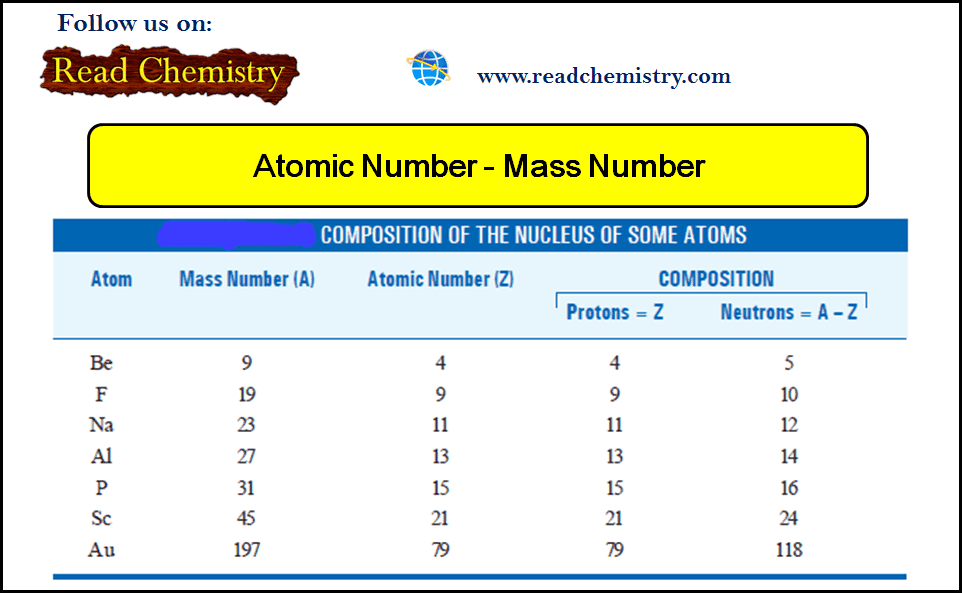
Atomic Number and Mass Number
– In this subject, we will discuss the Atomic Number and Mass Number Mosley’s Determination of Atomic Number – The…
Read More » -
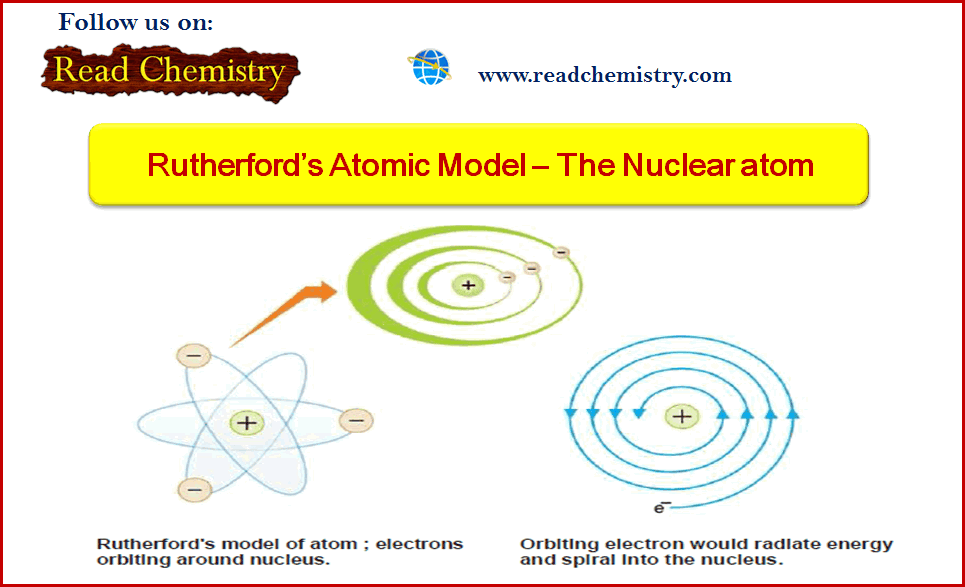
Rutherford’s Atomic Model (Experiment, Postulates, weakness)
– In this subject, we will discuss the Rutherford’s Atomic Model (Experiment, Postulates, weakness) Alpha particles – Alpha particles are…
Read More » -
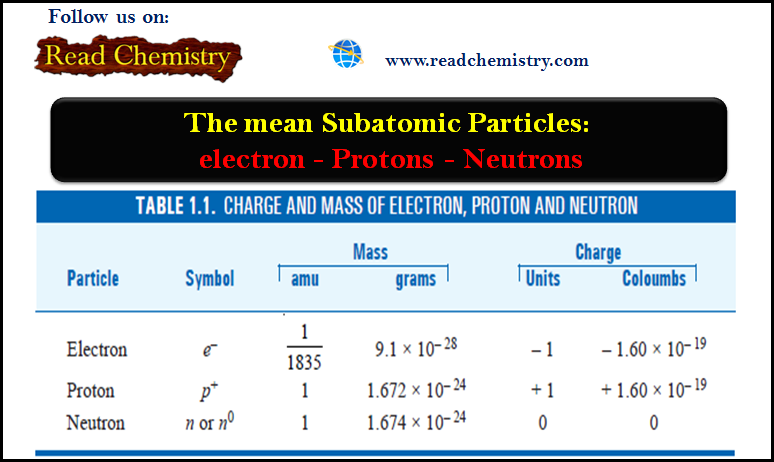
Subatomic Particles: Electrons, Protons, and Neutrons
– In this subject, we will discuss the Subatomic Particles: Electrons, Protons, and Neutrons Subatomic Particles – We have hitherto…
Read More » -
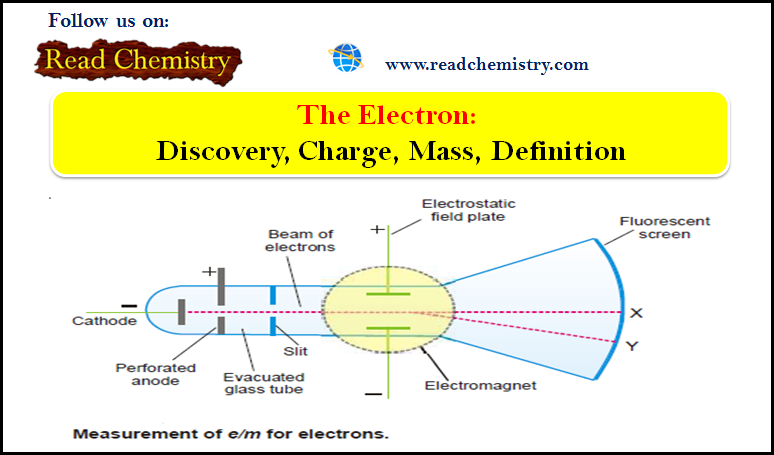
Electron: Discovery, Charge, Mass, Definition
Cathode Rays – The discovery of electron – The knowledge about the electron was derived as a result of the…
Read More » -
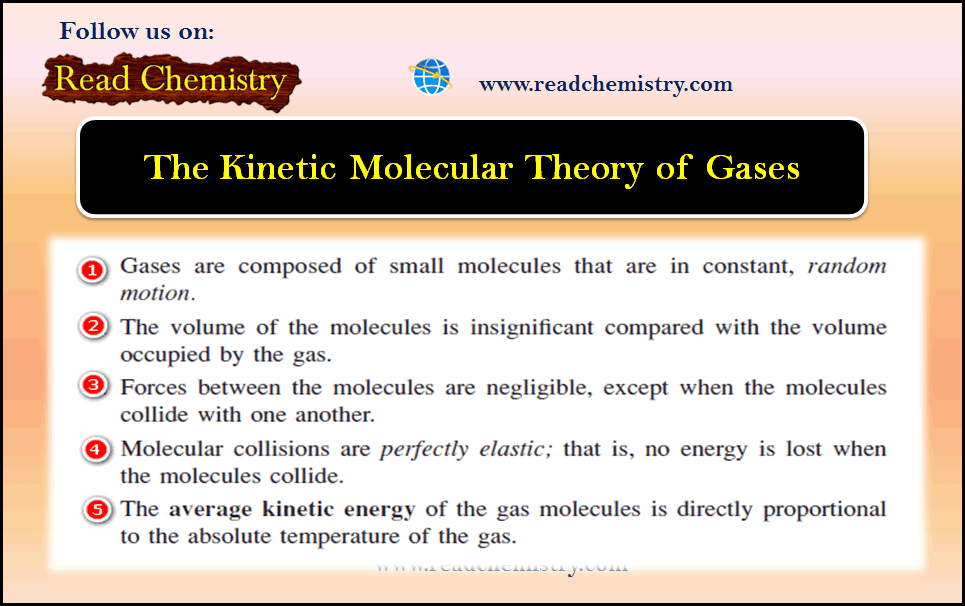
The Kinetic Molecular Theory of Gases
– In this subject, we will discuss the Kinetic Molecular Theory of Gases. Introduction to The Kinetic Molecular Theory –…
Read More » -
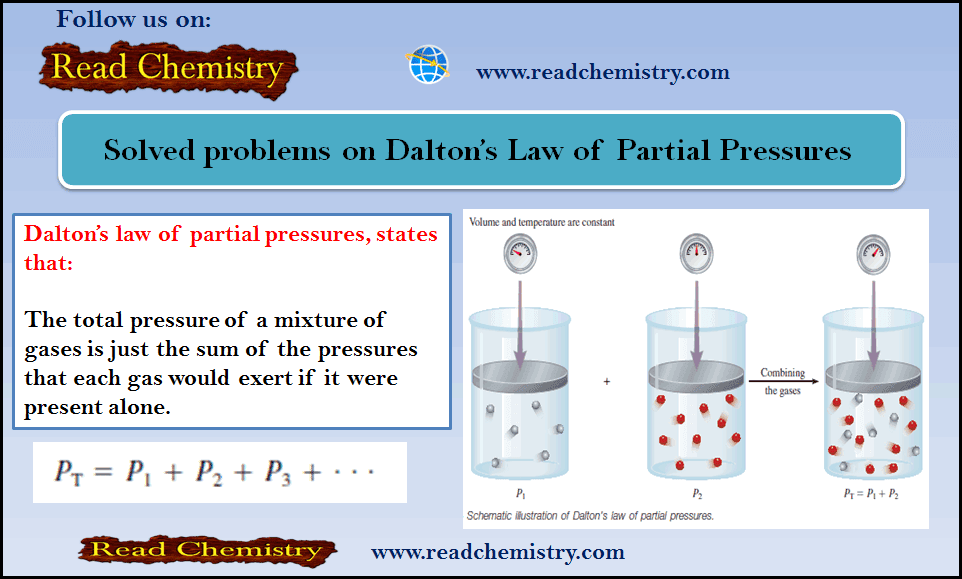
Solved problems: Dalton’s Law of Partial Pressures
– Before you solve these problems, you can read this subject for Dalton’s Law of Partial Pressures (Statement, Mathematical, Importance,…
Read More » -
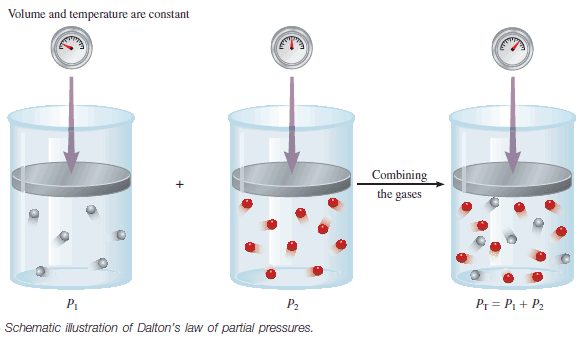
Dalton’s Law of Partial Pressures (Statement, Applications)
– In this subject, we will discuss Dalton’s Law of Partial Pressures (Statement, Mathematical, Importance, Application). Dalton’s Law of Partial Pressures…
Read More »