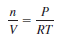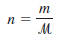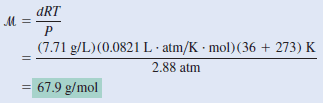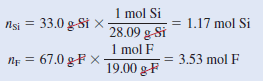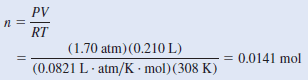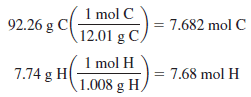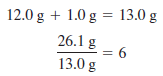Molar Mass of gas: Definition, Equation, Solved Examples
– In this subject, we will discuss the Molar Mass of gas (Definition, Equation, Solved Examples)
The Molar Mass of a gas
– From what we have seen so far, you may have the impression that the molar mass of a substance is found by examining its formula and summing the molar masses of its component atoms.
– However, this procedure works only if the actual formula of the substance is known.
– In practice, chemists often deal with substances of unknown or only partially defined composition.
– If the unknown substance is gaseous, its molar mass can nevertheless be found thanks to the ideal gas equation.
– All that is needed is an experimentally determined density value (or mass and volume data) for the gas at a known temperature and pressure.
Equation of the molar mass of gas
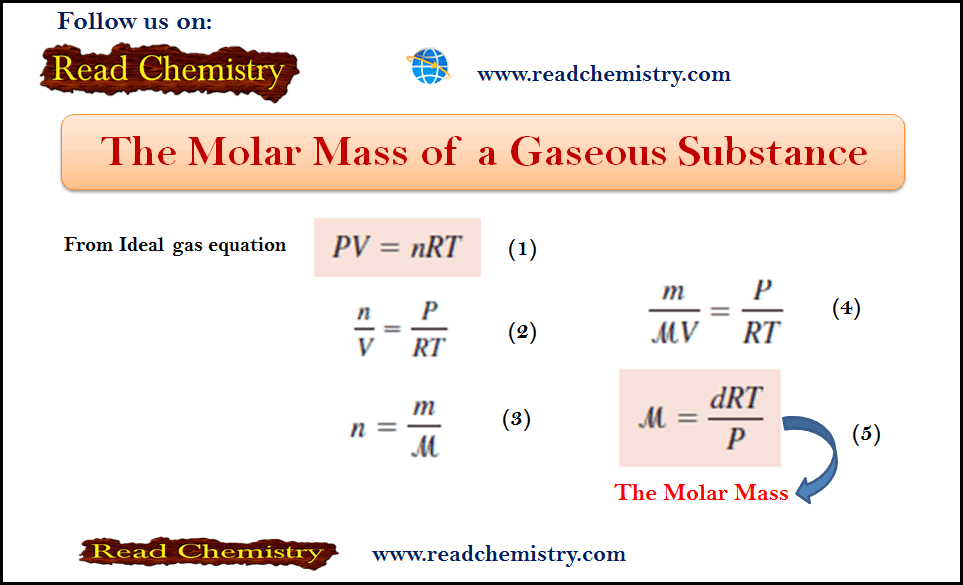
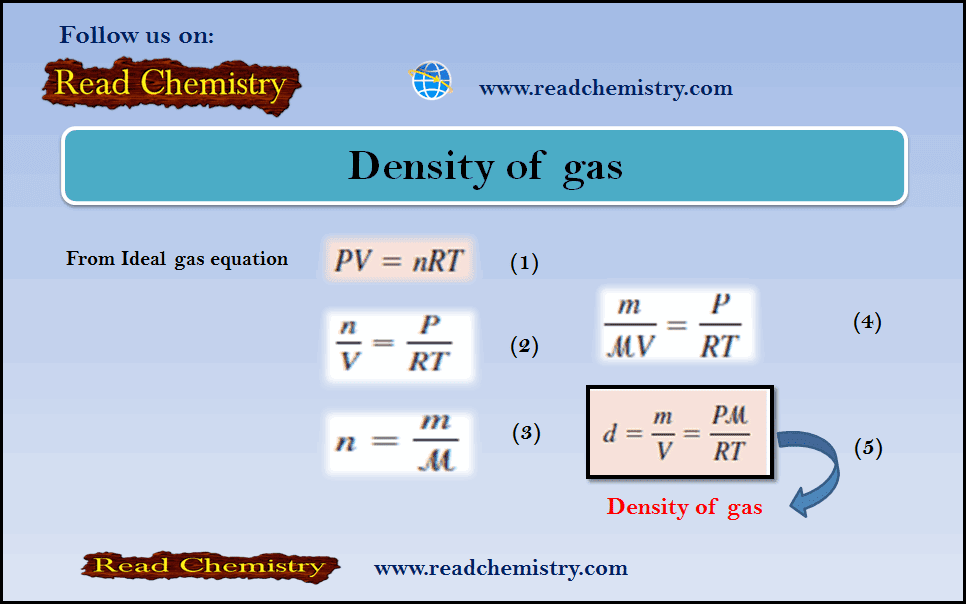
– The Molar Mass of a gas µ is derived as follows:
– From the ideal gas equation:
– If we rearrange the ideal gas equation, we can calculate the density of a gas:
– The number of moles of the gas, n, is given by:
– where m is the mass of the gas in grams and µ is its molar mass. Therefore
– If we rearrange the previous equation, we can calculate the Molar Mass of gas according to equation (1):
– In a typical experiment, a bulb of known volume is filled with the gaseous substance under study.
– The temperature and pressure of the gas sample are recorded, and the total mass of the bulb plus gas sample is determined (Figure 1).
– The bulb is then evacuated (emptied) and weighed again.
– The difference in mass is the mass of the gas.
– The density of the gas is equal to its mass divided by the volume of the bulb.
– Once we know the density of a gas, we can calculate the molar mass of the substance using Equation (1).
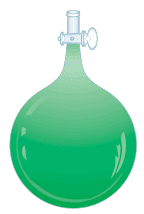
– Of course, a mass spectrometer would be the ideal instrument to determine the molar mass, but not every chemist can afford one.
– Problem (1) shows the density method for molar mass determination:
Solved problems on The Molar Mass of a gas
Problem (1)
The chemist has synthesized a greenish-yellow gaseous compound of chlorine and oxygen and finds that its density is 7.71 g/L at 36 oC and 2.88 atm. Calculate the molar mass of the compound and determine its molecular formula.
Strategy
– From Equation (1) we can calculate the molar mass of a gas if we know its density, temperature, and pressure.
– The molecular formula of the compound must be consistent with its molar mass.
– What temperature unit should we use?
Solution:
– From Equation (1):
Alternatively, we can solve for the molar mass by writing:
– From the given density we know there are 7.71 g of the gas in 1 L.
– The number of moles of the gas in this volume can be obtained from the ideal gas equation:
– Therefore, the molar mass is given by:
– We can determine the molecular formula of the compound by trial and error, using only the knowledge of the molar masses of chlorine (35.45 g) and oxygen (16.00 g).
– We know that a compound containing one Cl atom and one O atom would have a molar mass of 51.45 g, which is too low, while the molar mass of a compound made up of two Cl atoms and one O atom is 86.90 g, which is too high.
– Thus, the compound must contain one Cl atom and two O atoms and have the formula ClO2, which has a molar mass of 67.45 g.
– Because Equation (1) is derived from the ideal gas equation, we can also calculate the molar mass of gas using the ideal gas equation, as shown in problem (2).
Problem (2)
Chemical analysis of a gaseous compound showed that it contained 33.0 percent silicon (Si) and 67.0 percent fluorine (F) by mass. At 35°C, 0.210 L of the compound exerted a pressure of 1.70 atm. If the mass of 0.210 L of the compound was 2.38 g, calculate the molecular formula of the compound.
Strategy:
– This problem can be divided into two parts:
– First, it asks for the empirical formula of the compound from the percent by mass of Si and F.
– Second, the information provided enables us to calculate the molar mass of the compound and hence determine its molecular formula.
– What is the relationship between empirical molar mass and molar mass calculated from the molecular formula?
Solution:
– To calculate the empirical formula by assuming that we have 100 g of the compound, so the percentages are converted to grams.
– The number of moles of Si and F are given by:
– Therefore, the empirical formula is Si1.17 F3.53, or, dividing by the smaller subscript (1.17), we obtain SiF3.
– To calculate the molar mass of the compound, we need first to calculate the number of moles contained in 2.38 g of the compound.
– From the ideal gas equation:
– Because there are 2.38 g in 0.0141 mole of the compound, the mass in 1 mole, or the molar mass, is given by:
– The molar mass of the empirical formula SiF3 is 85.09 g.
– Recall that the ratio (molar mass/empirical molar mass) is always an integer (169/85.09 ~ 2).
– Therefore, the molecular formula of the compound must be (SiF3)2 or Si2 F6

Problem (3)
A 92.8-g sample of a pure gaseous substance occupies 29.5 L at and 1.25 atm. Calculate the molar mass of the gas.
Solution
– We recognize immediately that given the volume, temperature, and pressure of a gaseous substance, we can calculate the number of moles:
– The molar mass is simply the mass divided by the number of moles:
Problem (4)
Calculate the molecular formula of a gaseous compound composed of 92.26% C and 7.74% H if 0.507 g of the compound occupies 478 mL at 0.989 atm pressure.
Solution
– Even if we cannot see how to solve this problem completely at first glance, we can tell immediately that the empirical formula can be calculated from the percent composition and that the number of moles can be calculated from its pressure-volume-temperature data.
– The mole ratio of hydrogen to carbon is 1:1, so the empirical formula is CH.
– We can calculate the number of moles of gas present from the pressure, volume, and temperature data:
– The molar mass is the mass divided by the number of moles:
The mass of a mole of empirical formula units is:
– The mass of a mole of empirical formula units is C2H2
Reference:
- Chemistry / Raymond Chang, Williams College /(10th edition).
- Fundamentals of Chemistry / David E.Goldberg/(5th edition).