Electron: Discovery, Charge, Mass, Definition
Cathode Rays – The discovery of electron
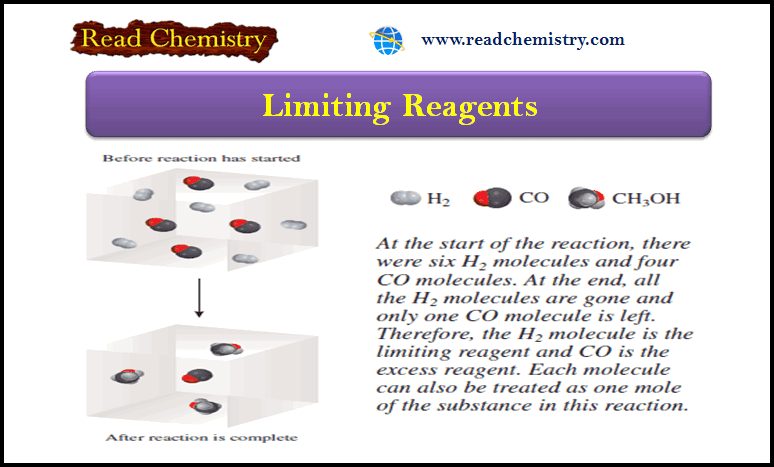
– The knowledge about the electron was derived as a result of the study of the electric discharge in the discharge tube (J.J. Thomson, 1896).
(1) The discharge tube consists of a glass tube with metal electrodes fused in the walls.
(2) Through a glass side-arm air can be drawn with a pump.
(3) The electrodes are connected to a source of high voltage (10,000 Volts) and the air partially evacuated.
(4) The electric discharge passes between the electrodes and the residual gas in the tube begins to glow.
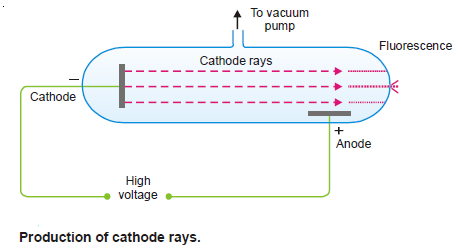
– If virtually all the gas is evacuated from within the tube, the glow is replaced by faintly luminous ‘rays’ which produce fluorescence on the glass at the end far from the cathode.
– The rays that proceed from the cathode and move away from it at right angles in straight lines are called Cathode Rays.
Properties of Cathode Rays
(1) They travel in straight lines away from the cathode and cast shadows of metallic objects placed in their path.
(2) Cathode rays cause the mechanical motion of a small pin-wheel placed in their path. Thus they possess kinetic energy and must be material particles.
(3) They produce fluorescence (a glow) when they strike the glass wall of the discharge tube.
(4) They heat up a metal foil to incandescence which they impinge upon.
(5) Cathode rays produce X-rays when they strike a metallic target.
(6) Cathode rays are deflected by the electric as well as the magnetic field in a way indicating that they are streams of minute particles carrying negative charge.
– By counterbalancing the effect of magnetic and electric field on cathode rays.
– Thomson was able to work out the ratio of the charge and mass (e/m) of the cathode particle.
– In SI units the value of (e/m) of cathode particles is – 1.76 × 188 coulombs per gram.
– As a result of several experiments, Thomson showed that the value of e/m of the cathode particle was the same regardless of both the gas and the metal of which the cathode was made.
– This proved that the particles making up the cathode rays were all identical and were constituent parts of the various atoms.
– Dutch Physicist H.A. Lorentz named them Electrons.
– Electrons are also obtained by the action of X-rays or ultraviolet light on metals and from heated filaments.
– These are also emitted as β-particles by radioactive substances.
– Thus it is concluded that electrons are a universal constituent of all atoms.
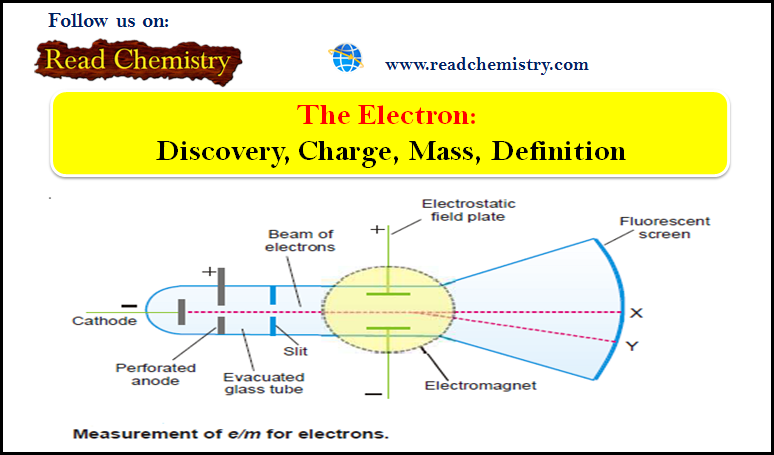
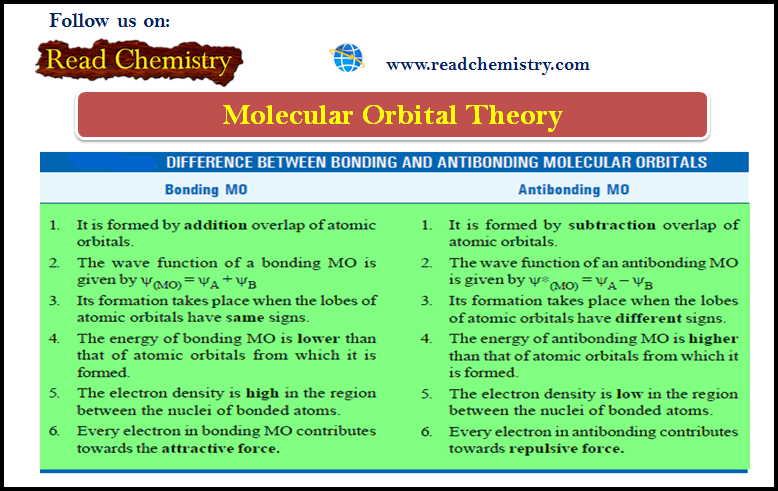
Measurement of e/m for electrons
– The ratio of charge to mass (e/m) for an electron was measured by J.J. Thomson (1897) using the apparatus shown in The following Figure:
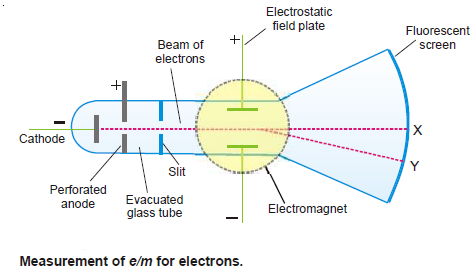
(1) Electrons produce a bright luminous spot at X on the fluorescent screen.
(2) A magnetic field is applied first and causes the electrons to be deflected in a circular path while the spot is shifted to Y.
(3) The radius of the circular path can be obtained from the dimensions of the apparatus, the current and number of turns in the coil of the electromagnet, and the angle of deflection of the spot.
(4) An electrostatic field of known strength is then applied so as to bring back the spot to its original position. Then from the strength of the electrostatic field and magnetic field, it is possible to calculate the velocity of the electrons.
– Equating magnetic force on the electron beam to centrifugal force:
- B = magnetic field strength
- v = velocity of electrons
- e = charge on the electron
- m = mass of the electron
- r = radius of the circular path of the electron in the magnetic field.
– This means:
– The value of (r) is obtained from the dimensions of the tube and the displacement of the electron spot on the fluorescent screen.
– When the electrostatic field strength and magnetic field strength are counterbalanced:
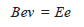
– where (E) is the strength of the electrostatic field.
– Thus
– If (E) and (B) are known, (v) can be calculated, and on substitution in equation (1), we get the value of (e/m):

– All the quantities on the right side of the equation can be determined experimentally.
– Using this procedure, the ratio e/m works out to be – 1.76 × 108 per gram.
– or
e/m for the electron = – 1.76 × 108 coulomb/g
Determination of the charge on an electron
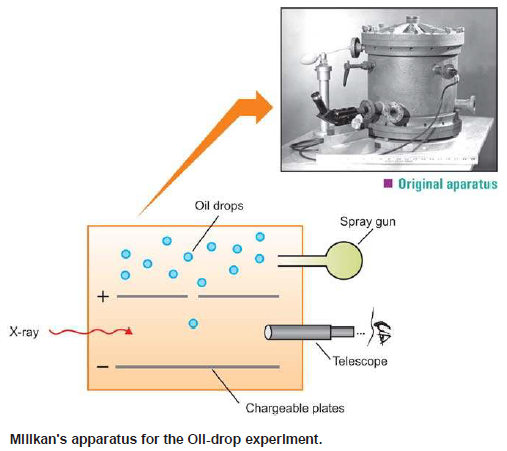
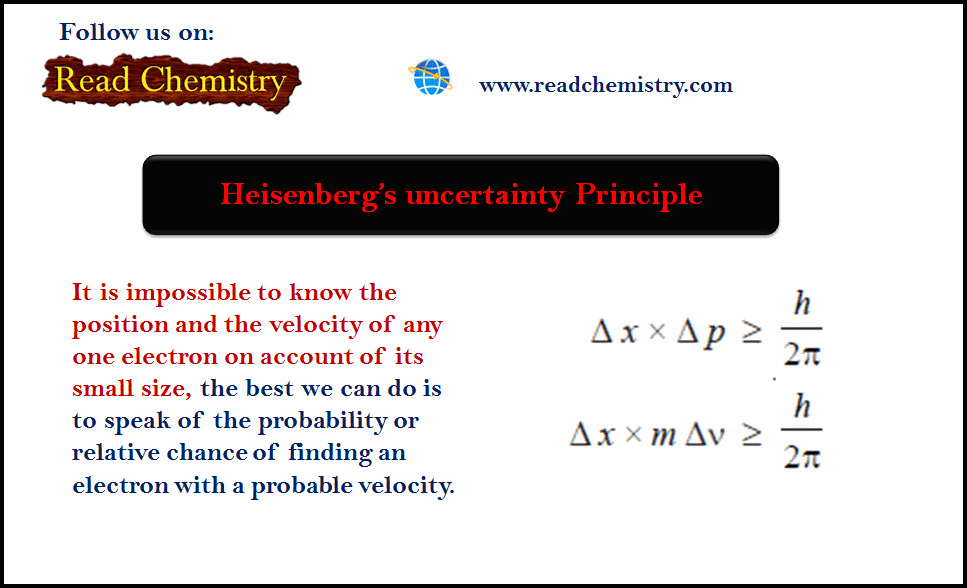
– The absolute value of the charge on an electron was measured by R.A. Milikan (1908) by what is known as Milikan’s Oil-drop Experiment.
– The apparatus used by Milikan is shown in The following figure:
(1) He sprayed oil droplets from an atomizer into the apparatus.
– An oil droplet falls through a hole in the upper plate
(2) The air between the plates is then exposed to X-rays which eject electrons from air molecules.
(3) Some of these electrons are captured by the oil droplet and it acquires a negative charge.
– When the plates are earthed, the droplet falls under the influence of gravity.
(4) He adjusted the strength of the electric field between the two charged plates so that a particular oil drop remained suspended, neither rising nor falling.
– At this point, the upward force due to the negative charge on the drop, just equaled the weight of the drop.
(5) As the X-rays struck the air molecules, electrons are produced.
– The drop captures one or more electrons and gets a negative charge, Q. Thus,
Q = ne
- n = number of electrons
- e = charge of the electron.
– From measurement with different drops, Milikan established that the electron has the charge – 1.60 × 10–19 coulombs.
Mass of Electron
– By using Thomson’s value of e/m and Millikan’s value of (e), the absolute mass of an electron can be found.
Mass of an Electron relative to H
– Avogadro number, the number of atoms in one gram atom of any element is 6.023 × 1023.
– From this, we can find the absolute mass of hydrogen atom.
– Mass of 6.023 × 1023 atoms of hydrogen = 1.008 g
– Thus an atom of hydrogen is 1835 times as heavy as an electron.
– In other words, the mass of an electron is 1/1835th of the mass of hydrogen atom.
Definition of an electron
– Having known the charge and mass of an electron, it can be defined as :
– The electron is a subatomic particle which bears charge – 1.60 × 10–19 coulomb and has mass 9.1 × 10–28 g.
– The electron is A particle which bears one unit negative charge and mass 1/1835th of a hydrogen atom.
– Since an electron has the smallest charge known, it was designated as unit charge by Thomson.
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolor edition.