System, Surroundings, and State: Definition – Overview
– In this subject, we will discuss the System, Surroundings, and State: Definition – Overview
What are the System and Surroundings?
– Imagine you have a container holding some material of interest to you, as in the Figure below.
– The container does a good job of separating the material from everything else.
– Imagine, too, that you want to make measurements of the properties of that material, independent from the measurements of everything else around it.
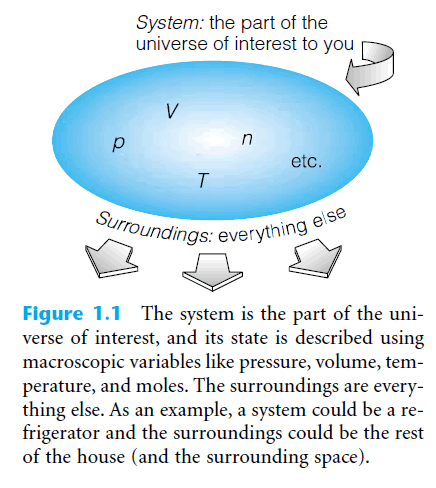
– The material of interest is defined as the system.
– The (everything else) is defined as the surroundings.
– These definitions have an important function because they specify what part of the universe we are interested in the system.
– Furthermore, using these definitions, we can immediately ask other questions: What interactions are there between the system and the surroundings? What is exchanged between the system and the surroundings?
– For now, we consider the system itself. How do we describe it? That depends on the system.
– For example, a glass of milk is described differently from the interior of a star.
– But for now, let us pick a simple system, chemically speaking.
What is the State?
– Consider a system that consists of a pure gas. How can we describe this system?
– Well, the gas has a certain volume, a certain pressure, a certain temperature, a certain chemical composition, a certain number of atoms or molecules, a certain chemical reactivity, and so on.
– If we can measure, or even dictate, the values of those descriptors, then we know everything we need to know about the properties of our system.
– We say that we know the state of our system.
Equilibrium between System and Surroundings
– If the state of the system shows no tendency to change, we say that the system is at equilibrium with the surroundings.
– The equilibrium condition is a fundamental consideration of thermodynamics.
– Although not all systems are at equilibrium, we almost always use equilibrium as a reference point for understanding the thermodynamics of a system.
– Equilibrium can be a difficult condition to define for a system.
– For example, a mixture of H2 and O2 gases may show no noticeable tendency to change, but it is not at equilibrium.
– It’s just that the reaction between these two gases is so slow at normal temperatures and in the absence of a catalyst that there is no perceptible change.
Energy of System
– There is one other characteristic of our system that we ought to know: its energy.
– The energy is related to all of the other measurables of our system (as the measurables are related to each other, as we will see shortly).
– The understanding of how the energy of a system relates to its other measurables is called thermodynamics (literally, heat movement).
– Although thermodynamics (thermo) ultimately deals with energy, it deals with other measurables too, and so the understanding of how those measurables relate to each other is an aspect of thermodynamics.
How do we define the state of the system?
– To begin, we focus on its physical description, as opposed to the chemical description.
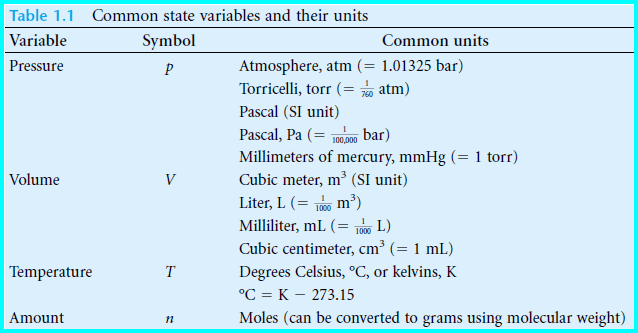
– We find that we can describe the macroscopic properties of our gaseous system using only a few observables: they are the system’s pressure, temperature, volume, and amount of matter (see Table below).
– These measurements are easily identifiable and have well-defined units.
(1) Volume (V)
– Volume has common units of liter, milliliter, or cubic centimeter.
– The cubic meter is the Système International (SI) unit of volume but these other units are commonly used as a matter of convenience.
(2) Pressure (P)
– Pressure has common units of atmosphere, torr, pascal (1 pascal = 1 N/m2 and is the SI unit for pressure), or bar.
– Volume and pressure also have obvious minimum values against which a scale can be based.
– Zero volume and zero pressure are both easily definable. The amount of material is similar.
– t is easy to specify an amount in a system, and having nothing in the system corresponds to an amount of zero.
(3) The temperature of a system (T)
– The temperature of a system has not always been an obvious measurement of a system, and the concept of a (minimum temperature) is relatively recent.
– In 1603, Galileo was the first to try to quantify changes in temperature with a water thermometer.
– Gabriel Daniel Fahrenheit devised the first widely accepted numerical temperature scale after developing a successful mercury thermometer in 1714, with zero set at the lowest temperature he could generate in his lab.
– Anders Celsius developed a different scale in 1742 in which the zero point was set at the freezing point of water. These are relative, not absolute, temperatures.
– Warmer and colder objects have a temperature value in these relative scales that is decided to these and other defined points in the scale.
– In both cases, temperatures lower than zero are possible and so the temperature of a system can sometimes be reported as a negative value.
Note: Volume, pressure, and amount cannot have a negative value, and later we define a temperature scale that cannot, either.
– Temperature is now considered a well-understood variable of a system.
Reference: Physical Chemistry /David W. Ball / Cleveland State University /2011.