Zeroth Law of Thermodynamics
– In this subject, we will discuss The Zeroth Law of Thermodynamics
Introduction to Zeroth Law of Thermodynamics
– Thermodynamics is based on a few statements called laws that have broad applications to physical and chemical systems.
– As simple as these laws are, it took many years of observation and experimentation before they were formulated and recognized as scientific laws.
– Three such statements that we will eventually discuss are the first, second, and third laws of thermodynamics.
– However, there is an even more fundamental idea that is usually assumed but rarely stated because it is so obvious.
– Occasionally this idea is referred to as the Zeroth law of thermodynamics since even the first law depends on it.
– It has to do with one of the variables that was introduced in the previous section, temperature.
What is temperature?
– Temperature is a measure of how much kinetic energy the particles of a system have.
– The higher the temperature, the more energy a system has, with all other variables defining the state of the system (volume, pressure, and so on) being the same.
– Since thermodynamics is in part the study of energy, temperature is a particularly important variable of a system.
– We must be careful when interpreting temperature, however.
– Temperature is not a form of energy. Instead, it is a parameter used to compare the amounts of energy of different systems.
Energy transfer between two systems
– Consider two systems, A and B, in which the temperature of A is greater than the temperature of B (Figure).
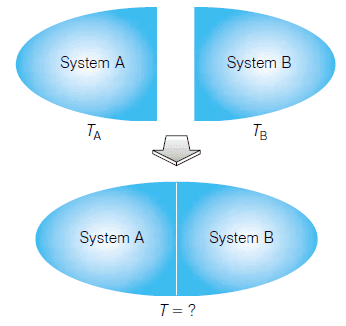
– Each is a closed system, which means that matter cannot move in or out of each system but energy can.
– The state of each system is defined by quantities like pressure, volume, and temperature.
– The two systems are brought together and physically joined but kept separate from each other, as shown.
– For example, two pieces of metal can be brought into contact with each other, or two containers of gas can be connected by a closed stopcock.
– Despite the connection, matter will not be exchanged between the two systems or with the surroundings.
– What about their temperatures, TA, and TB? What is always observed is that energy transfers from one system to another.
– As energy transfers between the two systems, the two temperatures change until the point where TA = TB. At that point, the two systems are said to be at thermal equilibrium.
– Energy may still transfer between the systems, but the net change in energy will be zero and the temperature will not change further.
– The establishment of thermal equilibrium is independent of the system size.
– It applies to large systems, small systems, and any combination of large and small systems.
– The transfer of energy from one system to another due to temperature differences is called heat.
– We say that heat has flowed from system A to system B.
– Further, if a third system C is in thermal equilibrium with system A, then TC = TA, and system C must be in thermal equilibrium with system B also.
– This idea can be expanded to include any number of systems, but the basic idea illustrated by three systems is summed up by a statement called the Zeroth law of thermodynamics:
What is the Zeroth Law of Thermodynamics?
– The zeroth law of thermodynamics states that:
If two systems (of any size) are in thermal equilibrium with each other and a third system is in thermal equilibrium with one of them, then it is in thermal equilibrium with the other also.
– The Zeroth law introduces a new idea.
– One of the variables that defines the state of our system (the state variables) changes its value.
– In this case, the temperature has changed.
– We are ultimately interested in how the state variables change and how these changes relate to the energy of our system.
– The final point for the system and its variables is the fact that the system does not remember its previous state.
– The state of the system is dictated by the values of the state variables, not their previous values or how they changed.
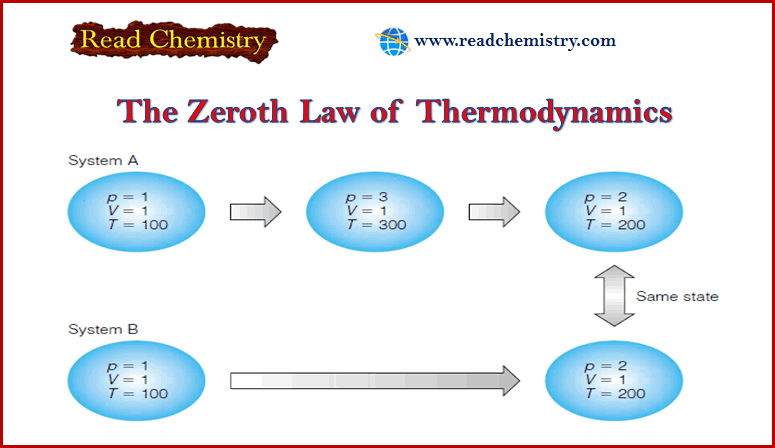
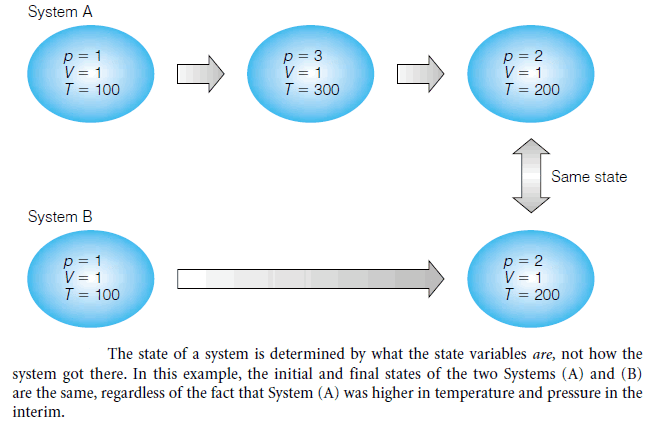
– Consider the two systems shown in Figure:
System A: goes to a higher temperature before settling on T = 200 temperature units.
System B: goes directly from the initial conditions to the final conditions.
– Therefore, the two states are the same.
– It does not matter that the first system was at a higher temperature; the state of the system is dictated by what the state variables are, not what they were, or how they got there.
Reference: Physical Chemistry /David W. Ball / Cleveland State University /2011.