– In this subject, we will discuss the Isotopic effects: definition, Applications
Definition of isotopes
Isotopes may be defined as :
(1) The atoms of an element that have the same number of protons and different numbers of neutrons are called Isotopes.
(2) The atoms of an element that have the same atomic number but different atomic masses or mass numbers.
Examples of isotopes
Isotopes of Hydrogen
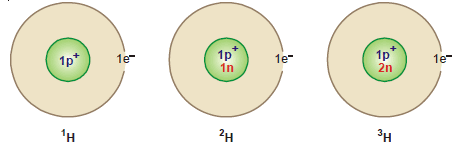
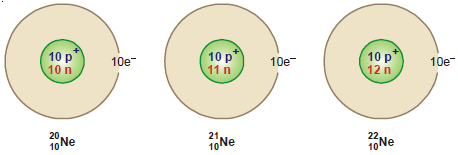
Isotopes of Neon
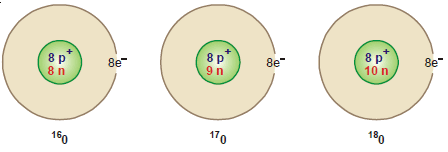
Oxygen
Isotopes of Chlorine
Isotopes of Uranium
 Isotopic effects
Isotopic effects
– Although in many respects the chemistry of the isotopes of an element is the same, there are significant differences between them due to differences in masses.
– Thus the physical properties of the compounds of each isotope of an element are distinctly different from those of others.
– Similarly, the reaction rates of the individual isotopes are also different.
– The differences in isotopes due to mass differences are termed Isotopic Effects.
– The isotopic effects show up clearly in the isotopes of hydrogen 1H1 and 2H1 (D).
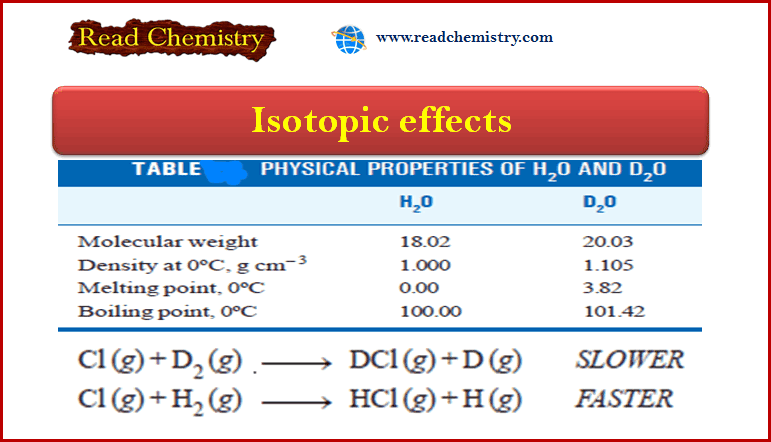
– The differences in the physical properties of water from ordinary hydrogen 1H1 and heavy hydrogen D are listed in Table
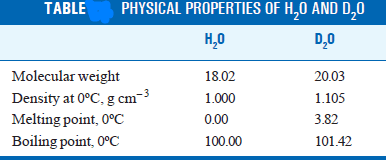
– The isotopic effects are also exhibited by the difference in the reaction rates of the two isotopes of hydrogen.
– Under similar conditions, the reaction of the heavy isotopes with chlorine is about six times slower than that of light isotopes.
– This difference in reaction rates is explained by the fact that the covalent bond formed by deuterium is slightly stronger than the corresponding bond with ordinary hydrogen.
– Hence a reaction that breaks a deuterium covalent bond is slower than the same reaction involving a bond to light hydrogen.
Applications of isotopic effects
– There are several useful applications of isotopic effects.
– One method of separation of isotopes of hydrogen depends on the fact that the electrolysis of heavy water (D2O) is slower than the electrolysis of normal water.
– Pure heavy water is a valuable by-product of the electrolysis of water since large quantities are required in nuclear reactors to moderate the rate of the uranium fission reaction.
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolor edition.
 Read Chemistry
Read Chemistry