Identification of Isotopes
– In this subject, we will discuss the Identification of Isotopes by Aston’s Mass Spectrograph and Dempster’s Mass Spectrograph
Identification of Isotopes
– The positive rays produced in a discharge tube consist of nuclei of atoms.
– The deflection of positive rays in an electric and magnetic field is proportional to e/m, the charge on the particle divided by its mass.
– The nuclei obtained from an element consisting of a mixture of isotopes will have the same positive charge and, therefore, their deflection will be inversely proportional to their masses.
– Thus with a suitable application of electric and magnetic fields, we can identify the isotopes present in a given element.
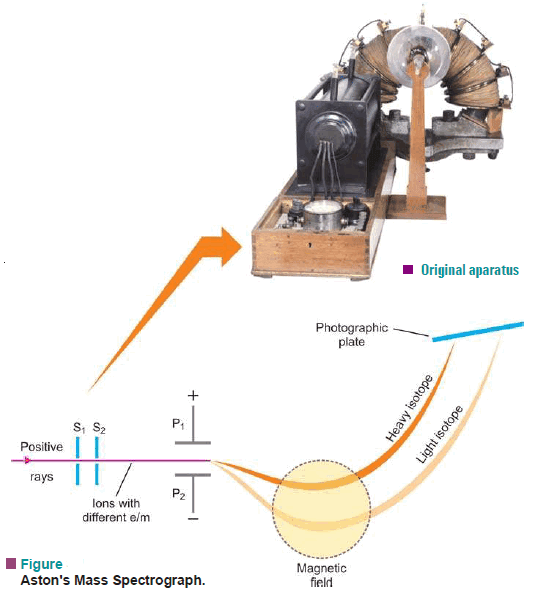
(1) Aston’s Mass Spectrograph
– Aston’s Mass Spectrograph is used for the Identification of Isotopes
– In 1919 F.W. Aston developed an instrument known as the Mass Spectrograph which can accurately sort out the positive ions of different isotopes of an element and determine their masses.
In this apparatus:
(1) a beam of positive rays obtained from a gaseous element in the discharge tube, is rendered into a fine ribbon by passing through slits S1 and S2.
(2) The fine beam consisting of positive ions of the various isotopes of the element is then sent between the electrostatically charged plates P1 and P2.
(3) Here the beam of positive ions is deflected down toward the negative plate.
(4) The slow-moving ions of the same isotope are deflected more than the faster ones which causes a broadening of the beam.
(5) Also, the heavier particles are deflected more (being slower) than the lighter ones and this brings about a separation of the various isotopes.
(6) The broadened beam of ions is then subjected to a magnetic field (shown by a dashed circle) at right angles to the plane of the charged plates and is thus sent in a direction opposite to that caused by the electrostatic field, slower particles again being deflected most.
(7) By adjustment of the two fields all ions of the same mass come to focus on the same point on the photographic plate where a sharp line is obtained.
(8) Thus each line recorded on the photographic plate shows the existence of a separate isotope.
– Further, the intensity of the line in comparison with the lines of other isotopes, gives the relative abundance of this particular isotope.
– The mass of a particle corresponding to a line produced on the photographic plate is determined by comparing it with a standard line produced by a particle of known mass (say, O+ = 16).
– For example, the examination of a sample of neon and chlorine by Aston’s Mass Spectrograph showed that they were made of Ne-20, Ne-22 and Cl-35, Cl-37 respectively.
– The intensities of their lines showed that the relative abundance was:
– Thus Aston’s Mass spectrograph not only helped in identifying the isotopes present in an element but also helped in determining the average atomic mass of a given element.
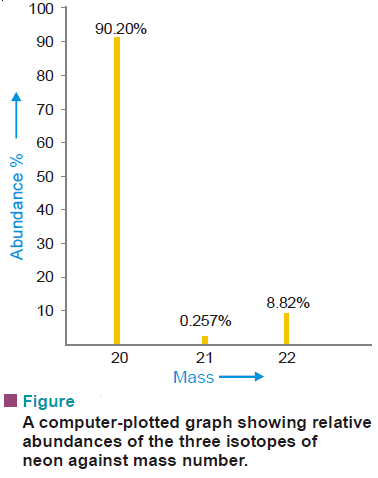
Example: A Sample of neon is found to consist of 20Ne, 21Ne, and 22Ne in the following percentages :
Calculate the atomic mass of neon.
Solution:
– The atomic mass of an ordinary isotopic mixture is the average of the determined atomic masses of individual isotopes. Thus
20 × 0.9092 = 18.18
21 × 0.0026 = 0.055
22 × 0.0882 = 1.94
= 20.18
The atomic mass of neon is 20.18
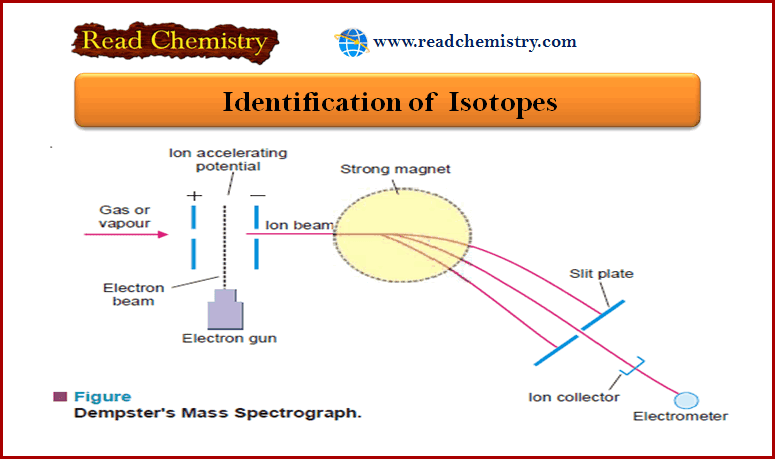
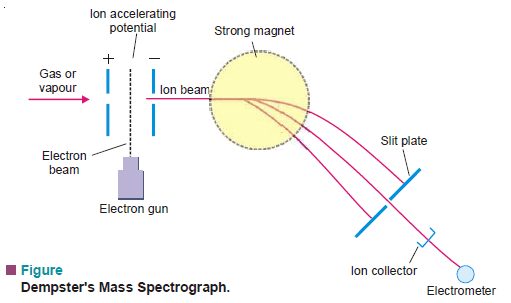
(2) Dempster’s Mass Spectrograph
– Dempster’s Mass Spectrograph is used for the Identification of Isotopes
In this apparatus:
(1) a slow stream of gas or vapor of the sample under examination is passed in between two perforated plates.
(2) Here it is bombarded by high-energy electrons shot out from an electron gun.
(3) The gas atoms are thus stripped of an electron and are converted to mono-positive ions (atom – e = ion 1+).
(4) When a potential of 500 to 2000 volts is applied between the perforated accelerating plates, the positive ions are strongly attracted to the negative plate.
(5) The beam of positive ions moving with accelerated speed then enters the magnetic field at right angles to its path and is thus made to move in a circular path.
– If V is the potential applied across the accelerating plates and e is the charge on each positive particle, the electrical energy is Ve.
– This is imparted to the particles as kinetic energy, 1/2 mv2. Thus,
– In the magnetic field of strength H, the magnetic force on the particle Hev, exactly balances the centrifugal force, mv2/r, r being the radius of the circular path. Thus,
– Eliminating ν between (1) and (2), we have
– e = the unit electrical charge, and r (depending on the particular apparatus) is constant.
– If during an experiment magnetic field H is kept the same, from the last equation it follows that
– Thus by adjusting the accelerating potential (V), particles of mass m can be made to fall on the collector plate.
– Each ion sets up a minute electric current which passes to the electrometer.
– The strength of the current thus measured, gives the relative abundance of the particles of mass m.
– Similarly, the particles of the other isotopes having different masses are made to fall on the collector and current strength is measured.
– The current strength in each case gives the relative abundance of the individual isotopes.
– By comparing the current strengths with an experiment performed with C-12 ion, the mass numbers of the various isotopes can be determined.
– In modern mass spectrographs, each ion strikes a detector, and the ion current is amplified and fed to a recorder.
– The recorder makes a graph showing relative abundance plotted against the mass number.
– A computer-plotted graph of neon isotopes is shown in the following Figure:
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolor edition.