Hybridization and Shapes of Molecules
Hybridization and Shapes of Molecules
– In the previous subject, we talked about the concept of Hybridization and the types of Hybridization, but in this subject, we will talk about Hybridization and Shapes of Molecules.
– Diatomic molecules must all be invariably linear but tri-and tetra-atomic molecules have several possible geometrical structures.
– In this subject, we will try to arrive at the accepted shapes of some common molecules in the pathway of the popular concept of hybrid orbitals.
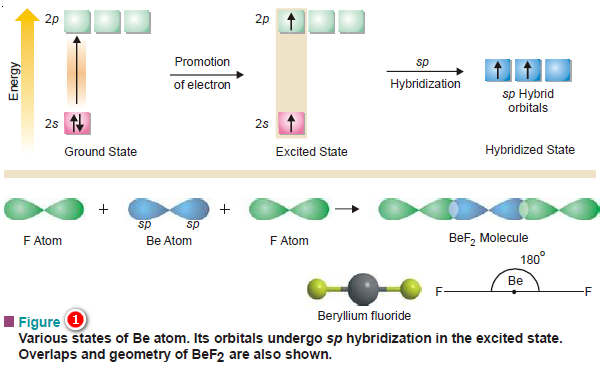
(1) Shape of BeF2 molecule
– An isolated Be atom in its ground state has the electronic configuration 1s2, 2s2.
– At first thought, one would expect Be to be chemically inert like He since it has all its orbitals completely filled (no bonding orbital).
– But Be behaves differently because its 2s orbital though complete, possesses another empty 2p level lying in the same shell. This is not so for He (1s2).
– Since orbitals on the same atom lying very close to one another in energy possess an unusual quality of mixing with one another additively forming new hybrid orbitals, there is ample scope for hybridization here.
– The Be atom, therefore, gets excited so that one of its 2s2 electrons is ‘promoted’ to the next available 2px orbital before the atom participates in chemical bonding.
– But this process requires energy which will be available from the heat of the reaction released at the time of bond formation.
– Now the excited atom acquires the structure 1s2, 2s1, 2px1.
– If at this stage the atom forms two bonds by suitable overlaps with two fluorine atoms, these bonds will not be identical, one involving the s and the other p orbitals.
– Thus the s and p orbitals first hybridize to form two new and completely equivalent sp hybrid orbitals.
– These hybrid orbitals of Be are now capable of forming bonds.
– The two sp hybrid orbitals overlap two 2p orbitals from two fluorine atoms in the ‘head on’ manner to form two σ bonds.
– The two sp orbitals being linear, lend a linear shape to BeF2 molecule with Be atom lying in the center and two F atoms on its either side so that F—Be—F angle is equal to 180º as shown in figure (1):
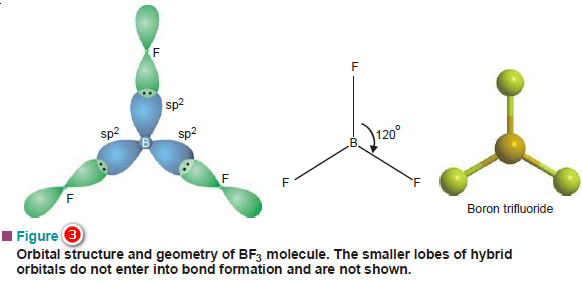
(2) Shape of BF3 molecule
– The orbital electronic configuration of Boron (B) is 1s2, 2s2, 2px1, 2py0, 2pz0.
– As there is only one bonding orbital 2px, B is expected to form only one bond.
– Boron, in fact, is known to form compounds such as BCl3, BF3, BH3, etc., indicating its capacity to form three bonds.
– What actually happens is that the two electrons of 2s orbital get unpaired when it is excited just like Be.
– One of these unpaired electrons thus gets promoted to the vacant 2pyorbital lying close to it.
– Thus in the excited state of Boron, there are three half-filled orbitals available for bonding.
– If as such it were to form bonds by overlap, the nature of these bonds would be different owing to their different types.
– One 2s and two 2p orbitals undergo sp2 hybridization giving three sp2 hybrid orbitals lying in one plane i.e., xy, and subtending an angle 120º.
– The equivalent hybrid orbitals can now enter into bond formation by overlapping with three 2p orbitals of three fluorine atoms as illustrated in Figure (3).
– Thus BF3 molecule is planar and each F—B—F angle is equal to 120º.
(3) Shapes of Carbon Compounds
– The carbon atom (1s2, 2s2, 2px1, 2py1, 2pz0) possesses two 2p bonding orbitals i.e., 2px and 2py, and can be expected to form only two bonds ordinarily.
– But in common practice, we come across compounds of carbon where it behaves as tetra-covalent.
– To remove the clash between the expected and the actual, the concept of hybridization comes to our rescue.
– Carbon can undergo three types of hybridization.
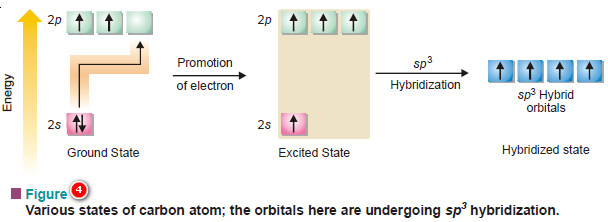
(a) sp3 Hybridization of Carbon
– It is proposed that from 2s orbital, being quite near in energy to 2p orbitals, one electron may be promoted to the vacant 2pz orbital thus obtaining the excited atom.
– At this stage, the carbon atom undoubtedly has four half-filled orbitals and can form four bonds.
– But by the strength of the argument extended in case of Be and B, it is assumed that the orbitals of carbon atom first undergo hybridization before forming bonds.
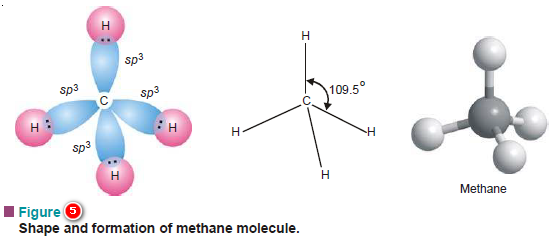
– In the excited atom all the four valence shell orbitals may mix up to give identical sp3 hybrid orbitals also four in number.
– Each of these four sp3 orbitals possesses one electron and overlaps with 1s orbitals of four H-atoms thus forming four equivalent bonds in methane molecule.
– Due to the tetrahedral disposition of sp3 hybrid orbitals, the orbitals are inclined at an angle of 109.5º.
– Thus all the HCH angles are equal to 109.5º.
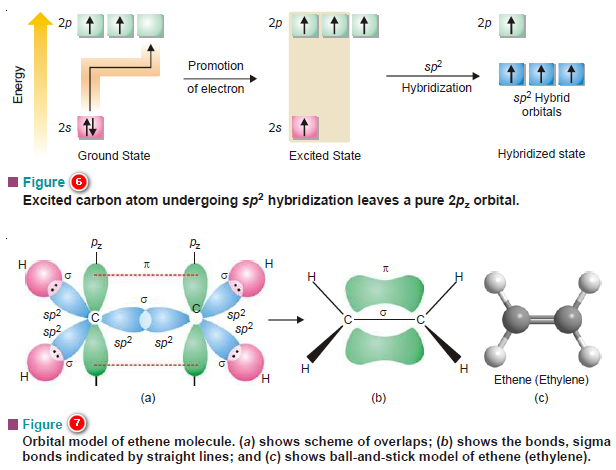
(b) sp2 Hybridization of Carbon
– When three out of the four valence orbitals hybridize, we have three sp2 hybrid orbitals lying in a plane and inclined at an angle of 120º.
– If 2s, 2px, and 2py orbitals of the excited carbon atom are hybridized, the new orbitals lie in the xy plane while the fourth pure 2pz orbital lies at right angles to the hybridized orbitals with its two lobes disposed above and below the plane of hybrid orbitals.
– Two such carbon atoms are involved in the formation of alkenes (compounds having double bonds).
– In the formation of ethene two carbon atoms (in sp2 hybridization state) form:
(1) one sigma bond by ‘head-on’ overlap of two sp2 orbitals contributed one each by the two atoms.
(2) The remaining two sp2 orbitals of each carbon form σ bonds with H atoms.
(3) The unhybridized 2pz orbitals of the two carbon atoms undergo a side-wise overlap forming a π bond.
– Thus the carbon to carbon double bond in ethene is made of one σ bond and one π bond.
– Since the energy of a π bond is less than that of a σ bond, the two bonds constituting the ethene molecule are not identical in strength. The molecule is a planar one.
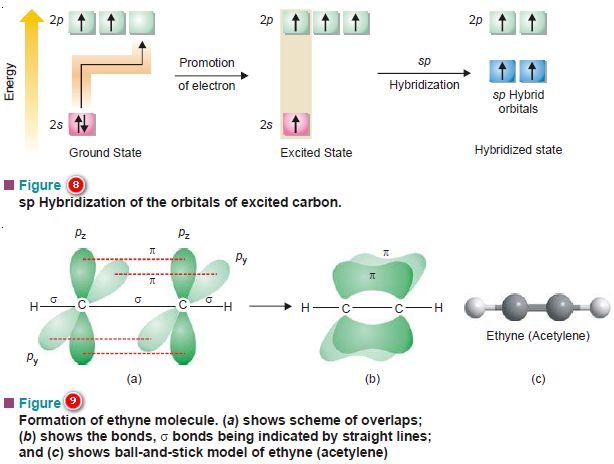
(c) sp Hybridization of Carbon
– This type of hybridization is met in alkynes (compounds having a triple bond between two carbons).
– Here one 2s and only one 2p orbital hybridize to form two equivalent colinear orbitals; the other two 2p orbitals remain undisturbed, both being perpendicular to the axis of hybrid orbitals.
– One of the two sp hybrid orbitals on each of the two carbons in ethyne molecule may be used in forming a σ bond between them.
– This leaves two pure 2p orbitals (2py and 2pz) on each carbon atom. Both these are mutually perpendicular to H–C–C–H nuclear axis, the C–H bonds being formed by the overlap of the remaining sp orbital with 1s orbitals of hydrogens (see Fig. 8).
– These pure 2p orbitals are capable of forming two π bonds by side-wise overlaps.
– Thus ethyne molecule contains one σ and a two π bonds between the two carbons and each carbon is linked with one H-atom through σ bonds.
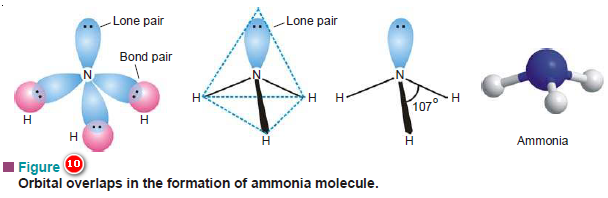
(4) Shape of Ammonia molecule, NH3
– There are three 2p bonding orbitals on the nitrogen atom ( 2px1, 2py1, 2pz1) It will form three σ bonds by overlapping with three 1s orbitals of the three H atoms.
– The orbital overlaps are shown in Fig (10).
– The orbital structure of NH3 has three orbital overlap axes inclined at an angle of 90º with N-atom as the origin.
– The four atoms in the molecule of NH3 do not lie in the same plane but form a pyramid at whose base are the three H-atoms and the N-atom is at the apex of the pyramid.
– What we can predict about the H–N–H bond angles is that they are 90º, the angle between the axes. However, this is erroneous and does not agree with the experimental value of 107º.
– The anomaly can be explained satisfactorily by employing:
(a) the concept of hybrid orbitals
(b) the electron pair interactions.
(a) sp3 Hybridization in ammonia molecule
– It is assumed that the valence orbitals of the central N-atom undergo hybridization before affecting overlaps with 1s orbitals of hydrogen.
– When the orbitals of the second energy level of N-atom (2s2, 2px1, 2py1, 2pz1) undergo sp3 hybridization, four new hybrid orbitals result.
– One of these will have two electrons (like the original atom) and is non-bonding while the other three have one electron each and can form bonds by overlap.
– Now these hybrid orbitals are tetrahedrally dispersed with an angle of 109.5º between them.
– After hybridization, let the 1s orbitals of three H-atoms overlap to form three σ bonds (Fig. 12a).
– The tetrahedral angle 109.5º is quite near the experimental value 107º, and a difference of 2.5º can be explained by taking into consideration the electron pair interactions.
(b) Electron pair repulsion
– We have seen that the symmetrical central N-atom has in its valence shell, three bond pairs (bp), each shared with one of the three H-atoms, and also a lone pair (lp) of electrons.
– The three bond pairs and one lone pair may get arranged tetrahedrally about the central atom.
– The central atom exercises different pulls on them.
– The lone pair is attracted more towards the N-atom than the bond pairs which belongs to the H-atoms and N-atom jointly.
– This is because of the fact that the lone pair belongs only to the N-atom and hence its electron cloud is more concentrated near the N-atom.
– The lone pair is, therefore, capable of exerting a greater repulsion on a bond pair than a bond pair can repel another bond pair.
– As a result, three bonds of ammonia molecule are forced slightly closer than in the normal tetrahedral arrangement.
– Therefore each of the HNH bond angles is 107º rather than the anticipated tetrahedral angle of 109.5º.
– It is also clear from the above reasoning that more the number of lone pairs greater will be their influence in decreasing the bond angles.
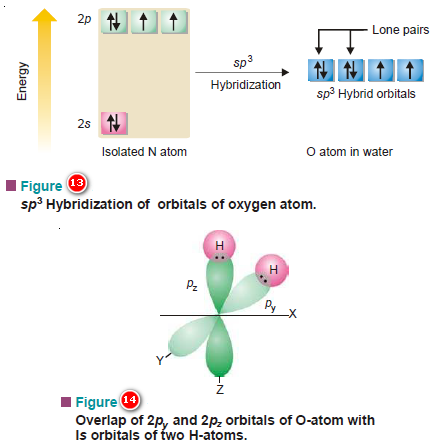
(5) Shape of Water molecule, H2O
– In the central oxygen atom of the molecule, there are two bonding orbitals ( 2py1 and 2pz1).
– These may overlap with 1s orbitals of two atoms of opposite spins.
– Each of these two overlaps results in the formation of a σ MO, giving two σ bonds in the molecule as a whole.
– The pictorial representation of the orbital overlaps is shown in Figure (14).
– Since the molecule involves two 2p orbitals at right angles and the bond established by an orbital retains the directional character of the bonding orbital, it is reasonable to expect the bond angle to the equal to 90º.
– But careful experiments reveal the HOH angle to be 104.3º rather than the predicted 90º.
– The discrepancy between the expected and the experimental values of the bond angle is best explained with the help of the hybridization concept.
– The valence orbitals i.e., of the second energy shell of the oxygen atom all hybridize giving four tetrahedrally dispersed sp3 hybrid orbitals.
– Two of these four hybrid orbitals have pairs of electrons with opposite spins and are non-bonding, while the rest two having one electron each are capable of bonding.
– They do so by overlaps with orbitals of two H atoms.
– An adequate guess of the HOH angle would be 109.5º, tetrahedral angle (Fig. 15 a & b).
– This is certainly in better agreement with the experimental value of 104.3º than our earlier contention of 90º on the basis of pure 2p orbital overlaps.
– But this is not all. The lone pair-bond pair repulsions have also to play their role.
– In water molecule there are two lone pairs in the vicinity of the central O-atom which has two bond pairs also.
– The repulsive forces operating are shown in Fig. 15 (c) above.
– Here we would expect the two lone pairs to repel each other more strongly than do a lone pair and a bond pair, and of course, even more strongly than two bond pairs.
– As a result, the two lone pairs of water force the two (O–H) bond pairs closer together than the one lone pair in case of ammonia forces together the three (N–H) bond pair.
– Thus the HOH angle is smaller (104.3º) than the HNH bond angles of 107º.
– In the light of the above discussions we can explain the molecular geometry of PH3, PCl3, NF3, H2S, etc.
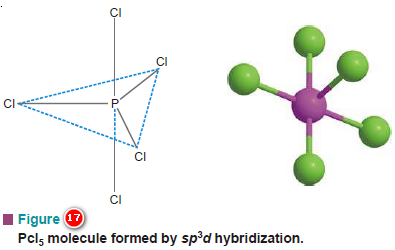
(6) Shape of Phosphorus pentachloride molecule, PCl5
– In PCl5 molecule, phosphorus is the central atom which has the electronic configuration 1s2, 2s2, 2p6, 3s2, 3p3, 3d0.
– In the ground state, it has only three bonding orbitals in the valence shell.
– One of the two 2s electrons uncouples itself and is promoted to the 3d orbital.
– The orbitals now hybridize in accordance with sp3d type as follows:
– Three of these five bonding orbitals lie in a plane inclined at an angle of 120º, while the other two are directed above and below the plane in a direction perpendicular to the plane, taking the shape of a trigonal bipyramid.
– These orbitals of phosphorus atom can overlap with those of five chlorine atoms forming the PCl5 molecule which will therefore have trigonal bipyramidal shape as shown in Fig.17.
– Here, some of the bond angles are 90º while other bonds have an angle of 120º between them.
– This geometry of the molecule explains the high reactivity of two of the five Cl atoms in PCl5 molecule.
(7) Shape of Sulphur hexafluoride molecule, SF6
– The sulphur atom has the electronic configuration 1s2, 2s2, 2p6, 3s2, 3p4, 3d0, showing the existence of only two bonding orbitals 3py and 3pz.
– But sulphur is known to be hexacovalent which may be explained by promoting one electron each from 3s and 3p orbitals to the vacant d orbitals of the valence shell.
– The orbitals of the excited atom then undergo sp3d2 hybridization to produce six equivalent hybrid orbitals each having one electron.
– These hybrid orbitals are now available for the overlap after getting octahedrally dispersed (four of them lying in one plane inclined at an angle of 90º while the other two directed above and below the plane perpendicularly).
– Six fluorine atoms (each having one 2p1 bonding orbital) may approach at the corners of the regular octahedron for overlap.
– The molecule SF6 formed will thus have octahedral structure as shown in Fig.19.
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolor edition