Polar and Nonpolar Molecules
– In this subject, we will discuss the Polar and Nonpolar Molecules.
Dipole moment
– The dipole moment is a physical property that can be measured experimentally.
– It is defined as the product of the magnitude of the charge in electrostatic units (esu) and the distance that separates them in centimeters (cm):
– The charges are typically on the order of 10-10 esu and the distances are on the order of 10-8 cm.
– Dipole moments, therefore, are typically on the order of 10-18 esu cm.
– For convenience, this unit, 1 × 10-18 esu cm, is defined as one debye and is abbreviated (D).
– The unit is named after Peter J. W. Debye, a chemist born in the Netherlands and who taught at Cornell University from 1936 to 1966.
– Debye won the Nobel Prize in Chemistry in 1936.)In SI units 1 D = 3.336 × 10-30 coulomb meter (C . m).
– If necessary, the length of the arrow can be used to indicate the magnitude of the dipole moment.
– Dipole moments are very useful quantities in accounting for the physical properties of compounds.
Polar and Nonpolar Molecules
– In the discussion of dipole moments, our attention was restricted to simple diatomic molecules.
– Any diatomic molecule in which the two atoms are different (and thus have different electronegativities) will, of necessity, have a dipole moment In general, a molecule with a dipole moment is a polar molecule.
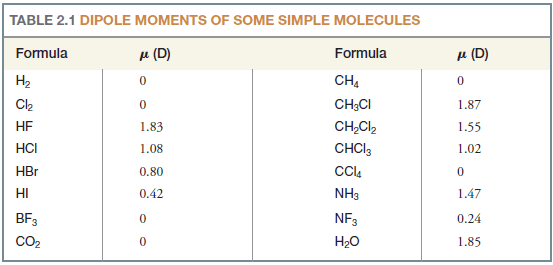
– If we examine Table (2.1), however, we find that several molecules (e.g., CCl4, CO2) consist of more than two atoms, and have polar bonds, but have no dipole moment.
– With our knowledge of the shapes of molecules, we can understand how this can occur.
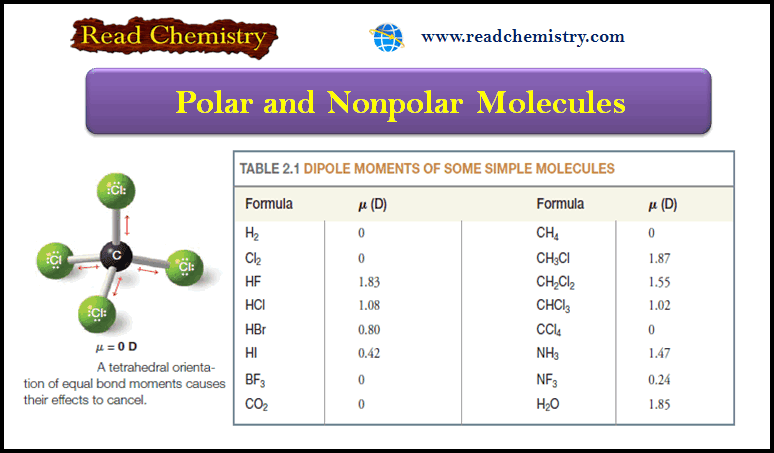
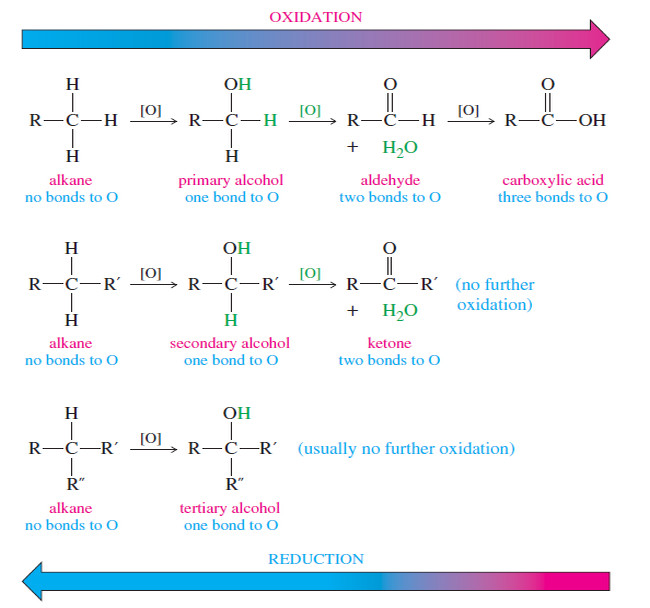
Carbon tetrachloride is Nonpolar
– Consider a molecule of carbon tetrachloride (CCl4).
– Because the electronegativity of chlorine is greater than that of carbon, each of the carbon chlorine bonds in CCl4 is polar.
– Each chlorine atom has a partial negative charge, and the carbon atom is considerably positive.
– Because a molecule of carbon tetrachloride is tetrahedral (Fig 1), however, the center of positive charge and the center of negative charge coincide, and the molecule has no net dipole moment.
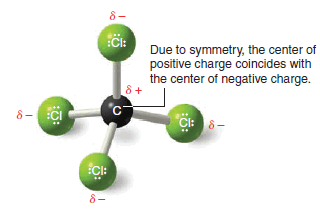
– This result can be illustrated in a slightly different way: if we use arrows (+→) to represent the direction of polarity of each bond, we get the arrangement of bond moments shown in Fig. 2.
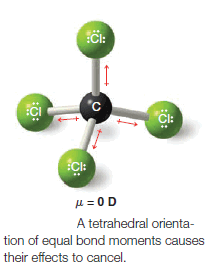
– Since the bond moments are vectors of equal magnitude arranged tetrahedrally, their effects cancel.
– Their vector sum is zero. The molecule has no net dipole moment.
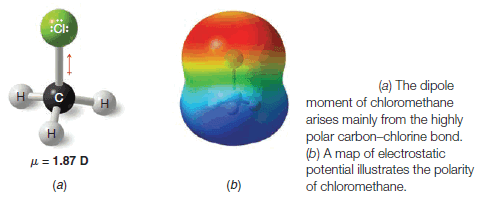
Chloromethane is Polar
– The chloromethane molecule (CH3Cl) has a net dipole moment of 1.87 D.
– Since carbon and hydrogen have electronegativities (Table 1) that are nearly the same, the contribution of three C-H bonds to the net dipole is negligible.
– The electronegativity difference between carbon and chlorine is large, however, and the highly polar C-Cl bond accounts for most of the dipole moment of CH3Cl (Fig. 3).
Solved Problem (1): Although molecules of CO2 have polar bonds (oxygen is more electronegative than carbon), carbon dioxide (Table 1) has no dipole moment. What can you conclude about the geometry of a carbon dioxide molecule?
Strategy and Answer:
– For a CO2 molecule to have a zero dipole moment, the bond moments of the two carbon-oxygen bonds must cancel each other.
– This can happen only if molecules of carbon dioxide are linear
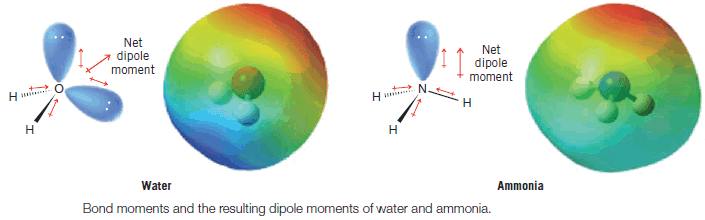
Water and Ammonia
– Unshared pairs of electrons make large contributions to the dipole moments of water and ammonia.
– Because an unshared pair has no other atom attached to it to partially neutralize its negative charge, an unshared electron pair contributes a large moment directed away from the central atom (Fig. 4). (The O-H and N-H moments are also appreciable.)
Dipole Moments in Alkenes
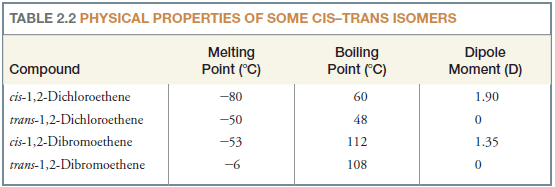
– Cis–trans isomers of alkenes have different physical properties.
– They have different melting points and boiling points, often cis-trans isomers differ markedly in the magnitude of their dipole moments.
– Table (2.2) summarizes some of the physical properties of two pairs of cis-trans isomers.
Solved Problem (2): Explain why cis-1,2-dichloroethene (Table 2) has a large dipole moment whereas trans-1,2-dichloroethene has a dipole moment equal to zero.
Strategy and Answer:
– If we examine the net dipole moments (shown in red) for the bond moments (black), we see that in trans-1,2-dichloroethene the bond moments cancel each other, whereas in cis-1,2 dichloroethene they augment each other.
Reference: Organic chemistry / T.W. Graham Solomons, Craig B.Fryhle, Scott A. Snyder, / ( eleventh edition) / 2014.