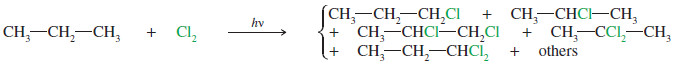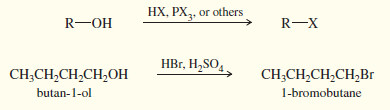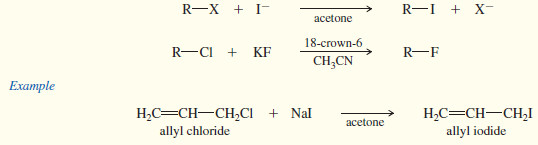Preparation of alkyl halides
Preparation of alkyl halides
– Most Methods of preparation of alkyl halides exploit the chemistry of functional groups we have not yet covered.
– For now, we review free-radical halogenation and only summarize other, often more useful, syntheses of alkyl halides.
– The other Methods of preparation of alkyl halides are discussed in subsequent subjects in Our website.
Free-Radical Halogenation
– free-radical halogenation is rarely an effective method for the preparation of alkyl halides.
– It usually produces mixtures of products because there are different kinds of hydrogen atoms that can be abstracted.
– Also, more than one halogen atom may react, giving multiple substitutions.
– For example, the chlorination of propane can give a messy mixture of products.
– In industry, free-radical halogenation is sometimes useful because the reagents are cheap, the mixture of products can be separated by distillation, and each of the individual products is sold separately.
– In a laboratory, however, we need a good yield of one particular product.
– Free-radical halogenation rarely provides good selectivity and yield, so it is seldom used in the laboratory.
– Laboratory syntheses using free-radical halogenation are generally limited to specialized compounds that give a single major product, such as the following examples
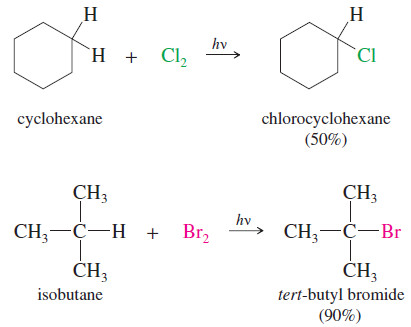
– All the hydrogen atoms in cyclohexane are equivalent, and free-radical chlorination gives a usable yield of chlorocyclohexane.
– Formation of dichlorides and trichlorides is possible, but these side reactions are controlled by using only a small amount of chlorine and an excess of cyclohexane.
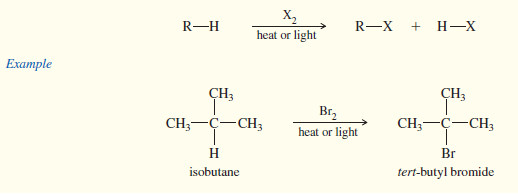
– Free-radical bromination is highly selective , and it gives good yields of products that have one type of hydrogen atom that is more reactive than the others.
– Isobutane has only one tertiary hydrogen atom, and this atom is preferentially abstracted to give a tertiary free radical.
– In general, however, we are not inclined to use free-radical halogenation in the laboratory because it tends to be plagued by mixtures of products.
Allylic Bromination
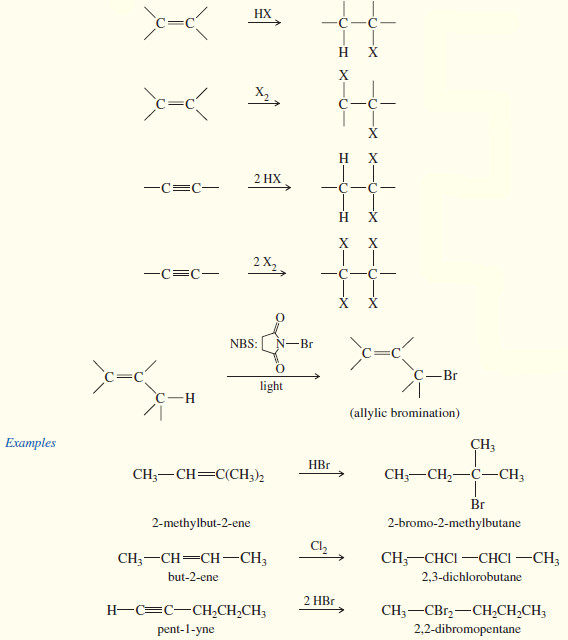
Although free-radical halogenation is a poor synthetic method in most cases, free-radical bromination of alkenes can be carried out in a highly selective manner.
– An allylic position is a carbon atom next to a carbon–carbon double bond. Allylic intermediates (cations, radicals, and anions) are stabilized by resonance with the double bond, allowing the charge or radical to be delocalized.
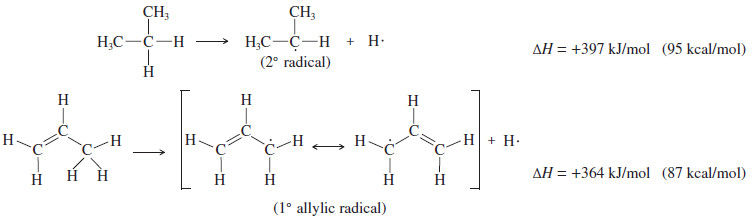
– The following bond dissociation enthalpies show that less energy is required to form a resonance-stabilized primary allylic radical than a typical secondary radical.
– Bromination is highly selective, with only the most stable radical being formed.
– If there is an allylic hydrogen, the allylic radical is usually the most stable of the radicals that might be formed.
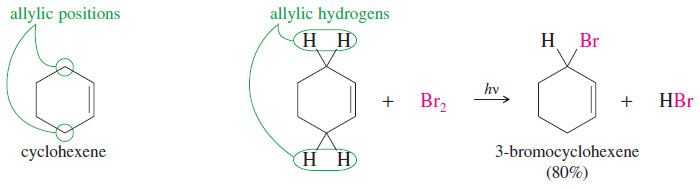
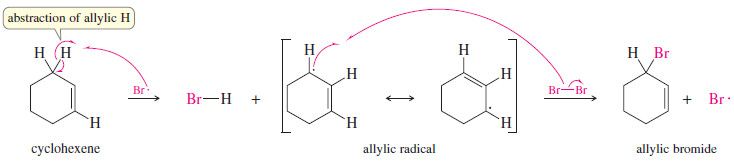
– For example, consider the free-radical bromination of cyclohexene.
– Under the right conditions, free-radical bromination of cyclohexene can give a good yield of 3-bromocyclohexene, where bromine has substituted for an allylic hydrogen on the carbon atom next to the double bond.
– The mechanism is similar to other free-radical halogenations.
– A bromine radical abstracts an allylic hydrogen atom to give a resonance-stabilized allylic radical.
– This radical reacts with Br2, regenerating a bromine radical that continues the chain reaction
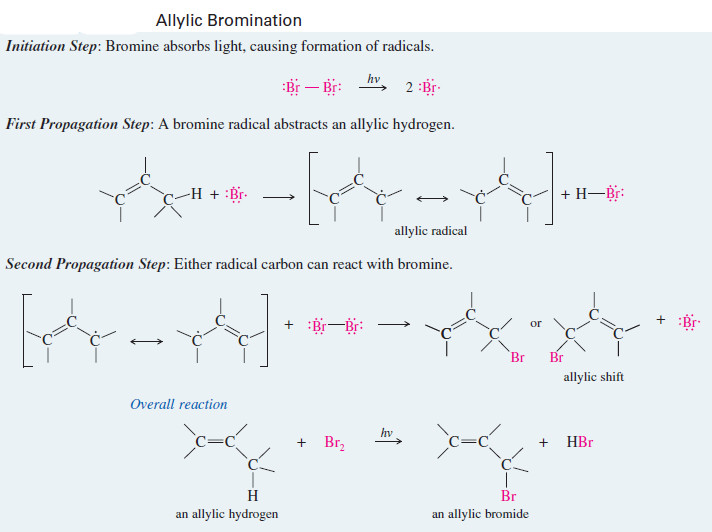
MECHANISM: Allylic Bromination
Initiation Step: Bromine absorbs light, causing formation of radicals.
First Propagation Step: A bromine radical abstracts an allylic hydrogen.
Second Propagation Step: Either radical carbon can react with bromine.
– The general mechanism for allylic bromination shows that either end of the resonance-stabilized allylic radical can react with bromine to give products.
– In one of the products, the bromine atom appears in the same position where the hydrogen atom was abstracted.
– The other product results from reaction at the carbon atom that bears the radical in the second resonance form of the allylic radical.
– This second compound is said to be the product of an allylic shift.
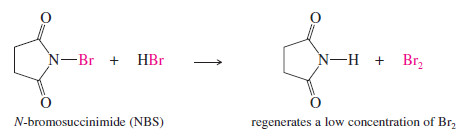
– For efficient allylic bromination, a large concentration of bromine must be avoided because bromine can also add to the double bond.
– N-Bromosuccinimide (NBS) is often used as the bromine source in free-radical brominations because it combines with the HBr side product to regenerate a constant low concentration of bromine.
– No additional bromine is needed because most samples of NBS contain traces of to initiate the reaction.
NBS also works well for brominating benzylic positions, next to an aromatic ring
SUMMARY Methods for Preparing Alkyl Halides
– Following is a brief summary of the most important methods of making alkyl halides.
– Many of them are more general and more useful than free-radical halogenation.
– Several of these methods are not discussed until later in the text
– They are listed here so that you can use this summary for reference throughout the course.
1. From alkanes: free-radical halogenation (synthetically useful only in certain cases
2. From alkenes and alkynes
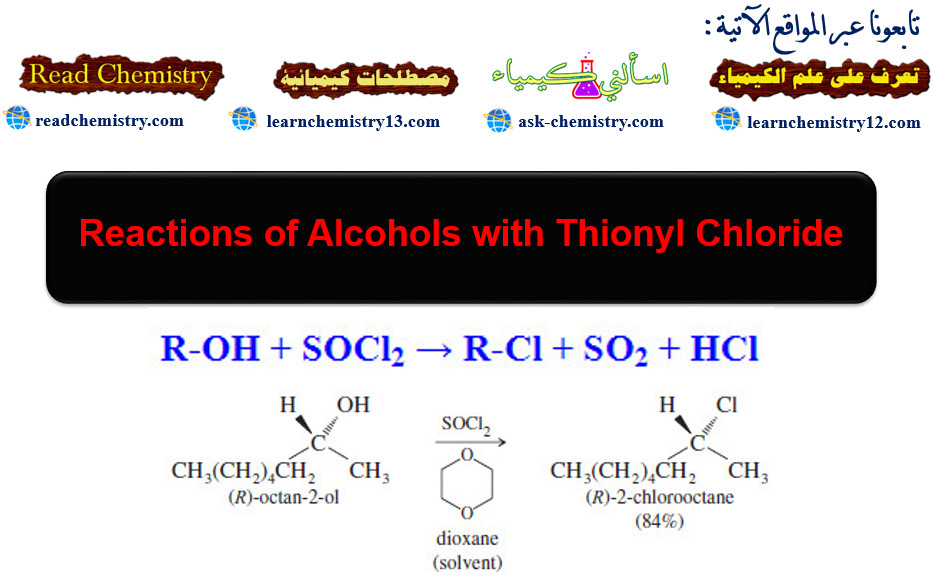
3. From alcohols
4. From other halides