Solubility of Organic Compounds
Solubility of Organic Compounds
– Intermolecular forces are of primary importance in explaining the solubility of substances.
– Dissolution of a solid in a liquid is, in many respects, like the melting of a solid.
– The orderly crystal structure of the solid is destroyed, and the result is the formation of a more disorderly arrangement of the molecules (or ions) in solution.
– In the process of dissolving, too, the molecules or ions must be separated from each other, and energy must be supplied for both changes.
– The energy required to overcome lattice energies and intermolecular or interionic attractions comes from the formation of new attractive forces between solute and solvent.
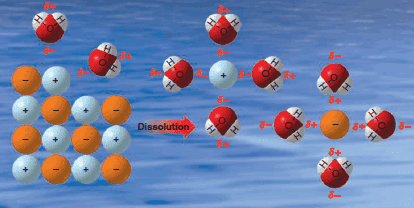
– Consider the dissolution of an ionic substance as an example:
(1) Here both the lattice energy and interionic attractions are large.
– We find that water and only a few other very polar solvents are capable of dissolving ionic compounds.
– These solvents dissolve ionic compounds by hydrating or solvating the ions (see Figure).
(2) Water molecules, by their great polarity as well as their very small, compact shape, can very effectively surround the individual ions as they are freed from the crystal surface.
(3) Positive ions are surrounded by water molecules with the negative end of the water dipole pointed toward the positive ion; negative ions are solvated in exactly the opposite way.
(4) Because water is highly polar, and because water is capable of forming strong hydrogen bonds, the ion-dipole forces of attraction are also large.
(5) The energy supplied by the formation of these forces is great enough to overcome both the lattice energy and interionic attractions of the crystal.
The general rule for the solubility of Organic Compounds
– A general rule for solubility is that “like dissolves like” in terms of comparable polarities:
(1) Polar and ionic solids are usually soluble in polar solvents.
(2) Polar liquids are usually miscible.
(3) Nonpolar solids are usually soluble in nonpolar solvents.
(4) Nonpolar liquids are usually miscible.
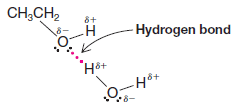
(5) Polar and nonpolar liquids, like oil and water, are usually not soluble to large extentsMethanol and water are miscible in all proportions; so too are mixtures of ethanol and water and mixtures of both propyl alcohols and water.
– In these cases, the alkyl groups of the alcohols are relatively small, and the molecules therefore resemble water more than they do an alkane.
– Another factor in understanding their solubility is that the molecules are capable of forming strong hydrogen bonds with each other:
Hydrophobic and Hydrophilic groups
– We often describe molecules or parts of molecules as being hydrophilic or hydrophobic.
– The alkyl groups of methanol, ethanol, and propanol are hydrophobic.
– Their hydroxyl groups are hydrophilic.
(a) Hydrophobic: means incompatible with water (hydro, water; phobic, fearing or avoiding).
(b) Hydrophilic: means compatible with water (philic, loving, or seeking).
– Decyl alcohol, with a chain of 10 carbon atoms, is a compound whose hydrophobic alkyl group overshadows its hydrophilic hydroxyl group in terms of water solubility
– An explanation for why nonpolar groups such as long alkane chains avoid an aqueous environment—that is, for the so-called hydrophobic effect—is complex.
– The most important factor seems to involve an unfavorable entropy change in the water.
– Entropy changes have to do with changes from a relatively ordered state to a more disordered one or the reverse.
– Changes from order to disorder are favorable, whereas changes from disorder to order are unfavorable.
– For a nonpolar hydrocarbon chain to be accommodated by water, the water molecules have to form a more ordered structure around the chain, and for this, the entropy change is unfavorable.
– The presence of a hydrophobic group and a hydrophilic group are essential components of soaps and detergents.
– The hydrophobic long carbon chains of soap or detergent embed themselves in the oily layer that typically surrounds the thing we want to wash away.
– The hydrophilic ionic groups at the ends of the chains are then left exposed on the surface and make the surface one that water molecules find attractive.
– Oil and water don’t mix, but now the oily layer looks like something ionic and the water can take it “right down the drain.”
Guidelines for Water Solubility
– Organic chemists usually define a compound as water-soluble if at least 3 g of the organic compound dissolves in 100 mL of water.
– We find that for compounds containing one hydrophilic group—and thus capable of forming strong hydrogen bonds—the following approximate guidelines hold:
(1) compounds with one to three carbon atoms are water soluble,
(2) compounds with four or five carbon atoms are borderline
(3) compounds with six carbon atoms or more are insoluble.
Important Notes
– When a compound contains more than one hydrophilic group, these guidelines do not apply.
– Polysaccharides, proteins, and nucleic acids all contain thousands of carbon atoms and many are water-soluble.
– They dissolve in water because they also contain thousands of hydrophilic groups.
Reference: Organic chemistry / T.W. Graham Solomons, Craig B.Fryhle, Scott A. Snyder, / ( eleventh edition) / 2014.