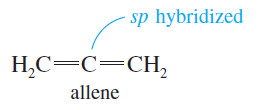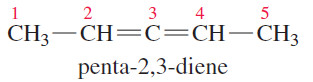Chiral Compounds without Asymmetric Atom
Chiral Compounds without Asymmetric Atoms
– Most chiral organic compounds have at least one asymmetric carbon atom.
– Some compounds are chiral because they have another asymmetric atom, such as phosphorus, sulfur, or nitrogen, serving as a chirality center.
– Some compounds are chiral even though they have no asymmetric atoms at all.
– In these types of compounds, special characteristics of the molecules’ shapes lend chirality to the structure.
Conformational Enantiomerism
– Some molecules are so bulky or so highly strained that they cannot easily convert from one chiral conformation to the mirror-image conformation.
– They cannot achieve the most symmetric conformation because it has too much steric strain or ring strain.
– Since these molecules are “locked” into a conformation, we must evaluate the individual locked-in conformation to determine whether the molecule is chiral.
– The following Figure shows three conformations of a sterically crowded derivative of biphenyl.
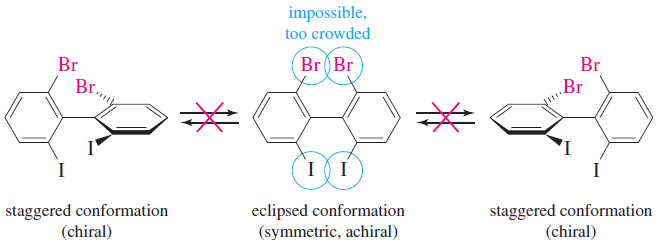
– The center drawing shows the molecule in its most symmetric conformation. This conformation is planar, and it has a mirror plane of symmetry.
– If the molecule could achieve this conformation, or even pass through it for an instant, it would not be optically active.
– This planar conformation is very high in energy, however, because the iodine and bromine atoms are too large to be forced so close together.
– The molecule is conformationally locked. It can exist only in one of the two staggered conformations shown on the left and right.
– These conformations are nonsuperimposable mirror images, and they do not interconvert. They are enantiomers, and they can be separated and isolated.
– Each of them is optically active, and they have equal and opposite specific rotations.
– Even a simple strained molecule can show conformational enantiomerism. trans- Cyclooctene is the smallest stable trans-cycloalkene, and it is strained.
– If transcyclooctene existed as a planar ring, even for an instant, it could not be chiral.
– Make a molecular model of trans-cyclooctene, however, and you will see that it cannot exist as a planar ring. Its ring is folded into the three-dimensional structure pictured in the following Figure.
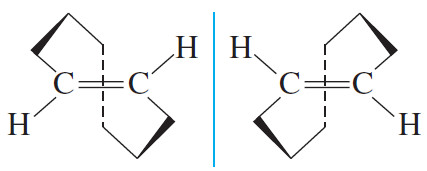
– The mirror image of this structure is different, and trans-cyclooctene is a chiral molecule.
– In fact, the enantiomers of trans-cyclooctene have been separated and characterized, and they are optically active.
Allenes
– Allenes are compounds that contain the unit, with two double bonds meeting at a single carbon atom.
– The parent compound, propadiene, has the common name allene.
– In allene, the central carbon atom is sp hybridized and linear, and the two outer carbon atoms are hybridized and trigonal.
– We might imagine that the whole molecule lies in a plane, but this is not correct.
– The central sp hybrid carbon atom must use different p orbitals to form the pi bonds with the two outer carbon atoms.
– The two unhybridized p orbitals on the sp hybrid carbon atom are perpendicular, so the two pi bonds must also be perpendicular.
– The following Figure shows the bonding and threedimensional structure of allene.
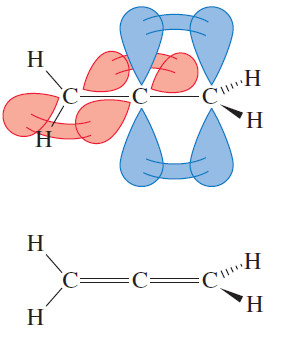
– Allene itself is achiral. If you make a model of its mirror image, you will find it identical with the original molecule.
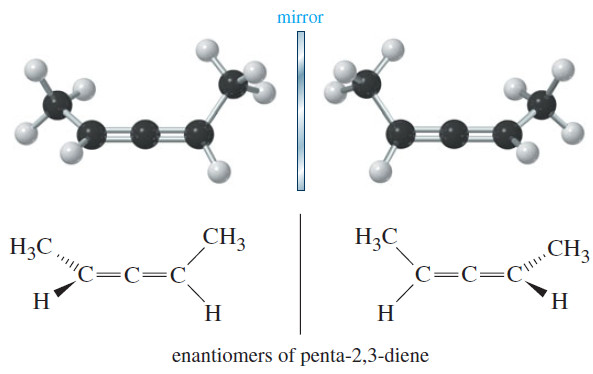
– If we add some substituents to allene, however, the molecule may be chiral. Make a model of the following compound:
– Carbon atom 3 is the sp hybrid allene-type carbon atom.
– Carbons 2 and 4 are both and planar, but their planes are perpendicular to each other.
– None of the carbon atoms is attached to four different atoms, so there is no asymmetric carbon atom.
– Nevertheless, penta-2,3-diene is chiral, as you should see from your models and from the following drawings of the enantiomers.