Spectroscopy of Ethers : IR, Mass, 13C NMR, 1H NMR
Spectroscopy of Ethers
– Here we will discuss Spectroscopy of Ethers: IR Spectroscopy, Mass Spectroscopy, 13C NMR amd 1H NMR.
Infrared Spectroscopy of Ethers
– Infrared spectra do not show obvious or reliable absorptions for ethers.
– Most ethers give a moderate to strong C-O stretch around 1000 to 1200 cm-1 (in the fingerprint region), but many compounds other than ethers give similar absorptions.
– Nevertheless, the IR spectrum can be useful because it shows the absence of carbonyl (C=O) groups and hydroxyl (O-H) groups.
– If the molecular formula contains an oxygen atom, the lack of carbonyl or hydroxyl absorptions in the IR suggests an ether.
Mass Spectrometry of Ethers
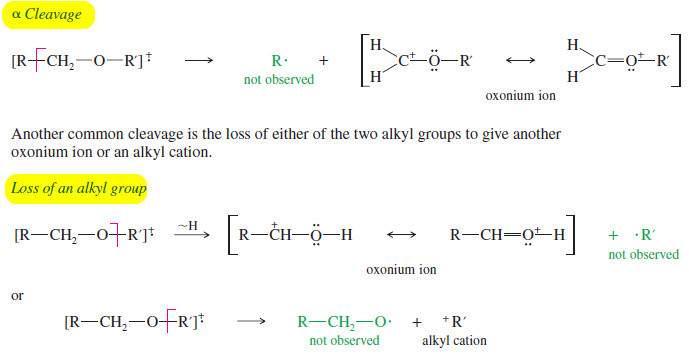
– The most common fragmentation of ethers is cleavage next to one of the carbon atoms bonded to oxygen.
– Because this carbon is alpha to the oxygen atom, this fragmentation is called α cleavage.
– The resulting oxonium ion (oxygen with three bonds and a positive charge) is resonance-stabilized by the nonbonding electrons on oxygen.
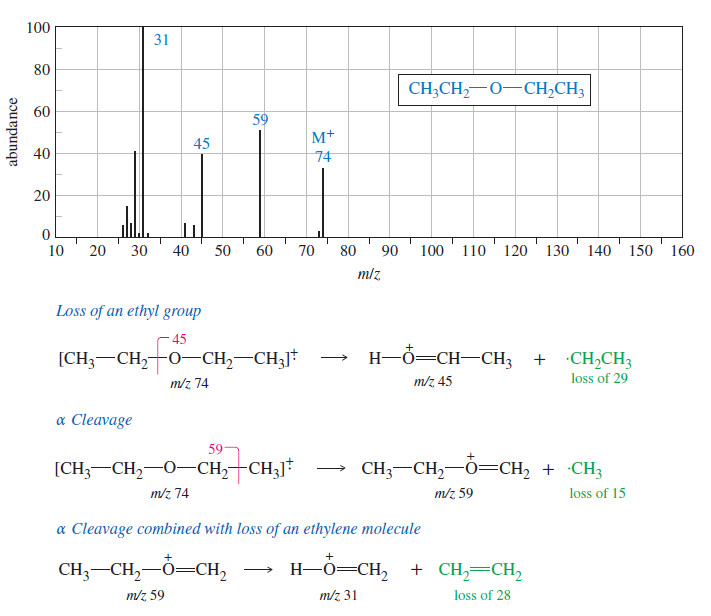
– The mass spectrum of diethyl ether appears in the following Figure:
– The four most abundant ions correspond to the molecular ion, loss of an ethyl group, α cleavage, and loss of an ethylene molecule combined with α cleavage.
– All these modes of cleavage form resonance-stabilized oxonium ions.
NMR Spectroscopy of Ethers
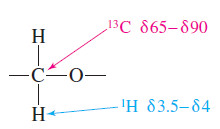
– In the 13C NMR spectrum, a carbon atom bonded to oxygen generally absorbs between δ 65 and δ 90.
– Protons on carbon atoms bonded to oxygen usually absorb at chemical shifts between δ 3.5 and δ 4 in the 1H NMR spectrum.
– Both alcohols and ethers have resonances in this range.
– If a compound containing C, H, and O has resonances in the correct range, and if there is no O-H stretch or C=O stretch in the IR spectrum, an ether is the most likely functional group.
References:
- Organic chemistry / L.G. Wade, Jr / 8th ed, 2013 / Pearson Education, Inc. USA.
- Fundamental of Organic Chemistry / John McMurry, Cornell University/ 8th ed, 2016 / Cengage Learningm, Inc. USA.
- Organic Chemistry / T.W. Graham Solomons, Craig B. Fryhle , Scott A. Snyder / 11 ed, 2014/ John Wiley & Sons, Inc. USA.