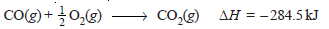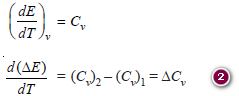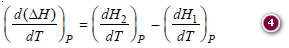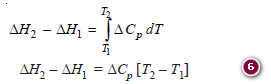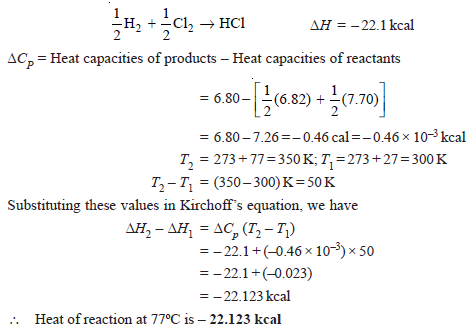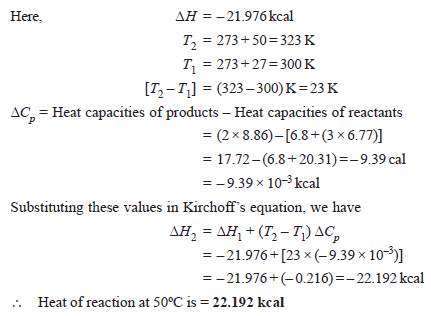Variation of heat of reaction with temperature
– In this subject, the Variation of heat of reaction with temperature will be discussed.
Heat of Reaction or Enthalpy of Reaction
– The heat of a reaction is simply the amount of heat absorbed or evolved in the reaction.
– We also know that the amount of heat absorbed or evolved at constant temperature and pressure is called enthalpy.
– Therefore the amount of heat change during a reaction at constant temperature and pressure may also be called enthalpy change.
– Its value depends upon the number of moles of the reactants which have reacted in the given chemical reaction.
– Heat of reaction may be defined as the amount of heat absorbed or evolved in a reaction when the number of moles of reactants as represented by the balanced chemical equation change completely into the products.
– For example, the heat change for the reaction of one mole of carbon monoxide with 0.5 mole of oxygen to form one mole of carbon dioxide is – 284.5 kJ.
– This means that 284.5 kJ of heat is evolved during the reaction and is the heat of reaction.
– It can be represented as:
– It is very important to note that heat of reaction varies with the change in temperature.
– we must mention the temperature at which the reaction is taking place.
– It is also convenient for comparison to fix up some temperature as standard or reference.
– According to the conventions prevalent in thermodynamics, the temperature of 298 K under a pressure of one atmosphere has been fixed as the standard state.
– The heat change accompanying a reaction taking place at 298 K and one atmospheric pressure is called the standard heat change or standard enthalpy change.
– It is denoted by ΔHº.
Variation Of Heat Of Reaction With Temperature
– The heat of reaction changes with change in temperature of a gas due to variation in its specific heat.
– The equations representing the variation of heat change of reaction with temperature are known as Kirchoff’s equations.
Kirchoff’s Equations
– At constant volume, the heat of reaction, ΔE, is given by the relation:
ΔE = E2 – E1
– where E1 and E2 are the internal energies of the reactants and products.
– Differentiating this equation with respect to temperature at constant volume, we get:
– But we have already seen that:
– where (Cv)2 and (Cv)1 are the heat capacities of the products and reactants respectively.
– So, Change in heat of reaction at constant volume per degree change in temperature is equal to the difference in heat capacities at constant volume of products and reactants.
– Integrating the above equation between temperatures T1 and T2, we have:
– where ΔE2 and ΔE1 are heats of reaction at temperatures T2 and T1 respectively.
– Similarly, at constant pressure the heat of reaction ΔH is given by the reaction:
ΔH = H2 – H1
– where H2 is the heat content (enthalpy) of the products and H1 being that of the reactants.
– Differentiating with respect to temperature at constant pressure, we have:
– According to the equation, we have:
– where (CP)2 and (CP)1 are the heat capacities of products and reactants respectively.
or
d (ΔH) = ΔCP × dT
– Change in heat of reaction at constant pressure per degree change of temperature is equal to the difference in heat capacities of products and reactants at constant pressure.
– Integrating the equation between temperature T1 and T2, we have
– The relations (2), (3), (5) and (6) were first derived by Kirchoff and are called Kirchoff’s equations.
– These equations may be used for calculating the heat of reaction at a given temperature when it is known at some other temperature and when the heat capacities of products and reactants are known.
Solved Problems on Variation of heat of reaction with temperature
Problem (1): The heat of reaction:
½ H2+ ½ Cl2 → HCl
at 27ºC is – 22.1 kcal. Calculate the heat of reaction at 77ºC. The molar heat capacities at constant pressure at 27ºC for hydrogen, chlorine, and HCl are 6.82, 7.70, and 6.80 cal mol–1 respectively.
Solution:
Problem (2): The heat of reaction:
N2+ 3H2 → 2NH3
at 27ºC was found to be –21.976 kcal. What will be the heat of reaction at 50ºC? The molar heat capacities at constant pressure and at 27ºC for nitrogen, hydrogen, and ammonia are 6.8, 6.77, and 8.86 cal mol–1degree–1.
Solution:
Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolor edition.