Colloids: Definition, History and Types
– In this topic, we will discuss the colloids: definition, History and Types
History of colloids
– Thomas Graham (1861) studied the ability of dissolved substances to diffuse into water across a permeable membrane.
– He observed that crystalline substances such as sugar, urea, and sodium chloride passed through the membrane, while others like glue, gelatin and gum arabic did not.
– The former he called crystalloids and the latter colloids (Greek, kolla = glue ; eidos = like).
– Graham thought that the difference in the behavior of ‘crystalloids’ and ‘colloids’ was due to the particle size.
– Later it was realised that any substance, regardless of its nature, could be converted into a colloid by subdividing it into particles of colloidal size.
What are colloids?
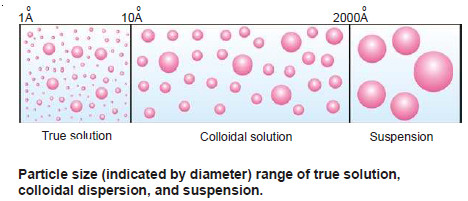
– In a true solution as sugar or salt in water, the solute particles are dispersed in the solvent as single molecules or ions. Thus the diameter of the dispersed particles ranges from 1Å to 10 Å.
– On the other hand, in a suspension as sand stirred into water, the dispersed particles are aggregates of millions of molecules. The diameter of these particles is of the order 2,000 Å or more.
– The colloidal solutions or colloidal dispersions are intermediate between true solutions and
suspensions.
– In other words, the diameter of the dispersed particles in a colloidal dispersion is more than that of the solute particles in a true solution and smaller than that of a suspension.
– When the diameter of the particles of a substance dispersed in a solvent ranges from about 10 Å to 2,000 Å, the system is termed a colloidal solution, colloidal dispersion, or simply a colloid.
– The material with particle size in the colloidal range is said to be in the colloidal state.
– The colloidal particles are not necessarily corpuscular in shape. In fact, these may be rod-like, disc-like, thin films, or long filaments.
– For matter in the form of corpuscles, the diameter gives a measure of the particle size. However, in other cases one of the dimensions (length, width and thickness) has to be in the colloidal range for the material to be classed as colloidal. Thus in a broader context we can say :
– A system with at least one dimension (length, width, or thickness) of the dispersed particles in the range 10 Å to 2,000 Å, is classed as a colloidal dispersion.
Types of colloidal systems
– As we have seen above, a colloidal system is made of two phases.
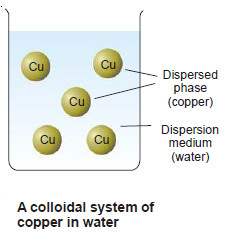
– The substance distributed as the colloidal particles is called the Dispersed phase.
– The second continuous phase in which the colloidal particles are dispersed is called the Dispersion medium.
– For example, for a colloidal solution of copper in water, copper particles constitute the dispersed phase and water the dispersion medium.
– As stated above, a colloidal system is made of a dispersed phase and the dispersion medium.
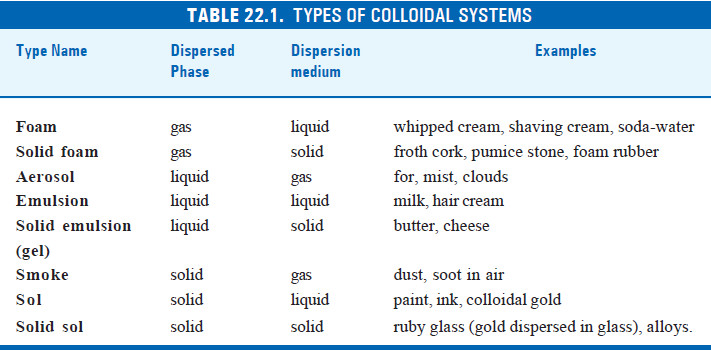
– Because either the dispersed phase or the dispersion medium can be a gas, liquid or solid, there are eight types of colloidal systems possible.
– A colloidal dispersion of one gas in another is not possible since the two gases would give a homogeneous molecular mixture.
– The various types of colloidal systems are listed in the following table:
– In the nexts tobics we will restrict our study mainly to the colloidal systems which consist of a solid substance dispersed in a liquid. These are frequently referred to as Sols or Colloidal solution.
– The colloidal solutions in water as the dispersion medium are termed Hydrosols or Aquasols.
– When the dispersions medium is alcohol or benzene, the sols are referred to as Alcosols and Benzosols respectively.