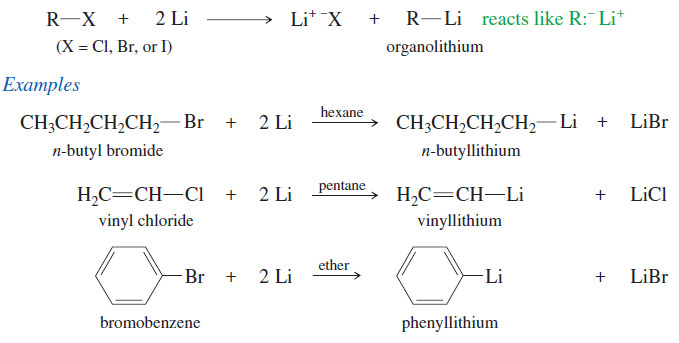
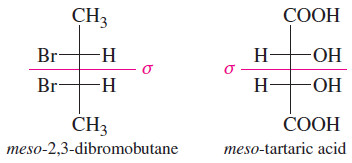Meso Compounds
Meso Compounds
– Compounds that are achiral even though they have asymmetric carbon atoms are called meso compounds.
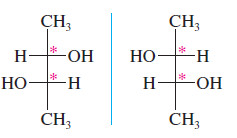
– The (2R,3S) isomer of 2,3-dibromobutane is a meso compound.
– most meso compounds have this kind of symmetric structure, with two similar halves of the molecule having opposite configurations.
– In speaking of the two diastereomers of 2,3-dibromobutane, the symmetric one is called the meso diastereomer, and the chiral one is called the (±) diastereomer, since one enantiomer is (+) and the other is (-)
Definition of Meso Compound
– Meso Compound is An achiral compound that has chirality centers (usually asymmetric carbons).
– The term meso (Greek, “middle”) was used to describe an achiral member of a set of diastereomers, some of which are chiral.
– The optically inactive isomer seemed to be in the “middle” between the dextrorotatory and levorotatory isomers.
– The definition just given (“an achiral compound with chirality centers”) is nearly as complete, and more easily applied, especially when you remember that chirality centers are usually asymmetric carbon atoms.
– We have already seen other meso compounds, although we have not yet called them that.
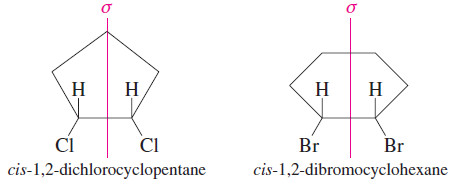
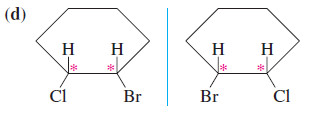
– For example, the cis isomer of 1,2 dichlorocyclopentane has two asymmetric carbon atoms, yet it is achiral.
– Thus it is a meso compound. cis-1,2-Dibromocyclohexane is not symmetric in its chair conformation, but it consists of equal amounts of two enantiomeric chair conformations in a rapid equilibrium.
– We are justified in looking at the molecule in its symmetric flat conformation to show that it is achiral and meso.
– For acyclic compounds, the Fischer projection helps to show the symmetry of meso compounds.
Solved problems on Meso Compound
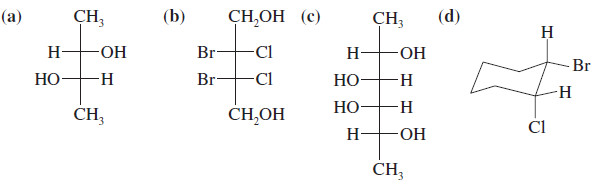
Problem (1): Determine which of the following compounds are chiral. Star(*) any asymmetric carbon atoms, and draw in any mirror planes. Label any meso compounds. (Use your molecular models to follow along.)
Solution:
– This compound does not have a plane of symmetry, and we suspect that it is chiral.
– Drawing the mirror image shows that it is nonsuperimposable on the original structure. These are the enantiomers of a chiral compound.
– Both (b) and (c) have mirror planes of symmetry and are achiral.
– Because they have asymmetric carbon atoms yet are achiral, they are meso.
– Drawing this compound in its most symmetric conformation (flat) shows that it does not have a mirror plane of symmetry.
– When we draw the mirror image, it is found to be an enantiomer.
Problem (2): One source defines a meso compound as “an achiral compound with stereocenters.” Why is this a poor definition?
Solution:
– A stereocenter is an atom at which the interchange of two groups gives a stereoisomer.
– Stereocenters include both chirality centers and double-bonded carbons giving rise to cis-trans isomers.
– For example, the isomers of but-2-ene are achiral and they contain stereocenters (circled), so they would meet this definition.
– They have no chiral diastereomers, however, so they are not correctly called meso.