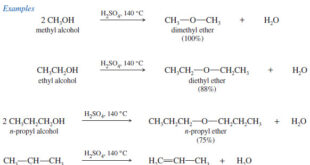
Conformational Analysis of Butane
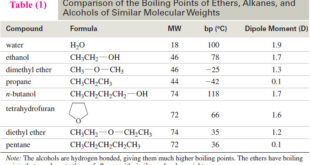
** Now let us consider rotation about the C2—C3 bond of butane. The barriers to rotation about the C2—C3 bond in butane are larger than for rotation about the C—C bond in ethane, but still not large enough to prevent the rotations that lead to all possible butane conformers.
** The factors involved in barriers to bond rotation are together called torsional strain and include the repulsive interactions called steric hindrance between electron clouds of the bonded groups.
** In butane, torsional strain results from steric hindrance between the terminal methyl groups and hydrogen atoms at C-2 and C-3 and from steric hindrance directly between the two methyl groups.
** These interactions result in six important conformers of butane, shown as I–VI below.
** The anti conformation (I) does not have torsional strain from steric hindrance because the groups are staggered and the methyl groups are far apart. The anti conformation is the most stable.
** The methyl groups in the gauche conformations III and V are close enough to each other that the dispersion forces between them are repulsive; the electron clouds of the two groups are so close that they repel each other. This repulsion causes the gauche conformations to have approximately 3.8 kJ mol-1 more energy than the anti conformation.
** The eclipsed conformations (II, IV, and VI) represent energy maxima in the potential energy diagram (Fig blow). Eclipsed conformations II and VI have repulsive dispersion forces arising from the eclipsed methyl groups and hydrogen atoms. Eclipsed conformation IV has the greatest energy of all because of the added large repulsive dispersion forces between the eclipsed methyl groups as compared to II and VI.
** Although the barriers to rotation in a butane molecule are larger than those of an ethane molecule, they are still far too small to permit isolation of the gauche and anti conformations at normal temperatures. Only at extremely low temperatures would the molecules have insufficient energies to surmount these barriers.
Figure: Energy changes that arise from rotation about the C2 – C3 bond of butane
We saw earlier that dispersion forces can be attractive. Here, however, we find that they can also be repulsive, leading to steric hindrance. Whether dispersion interactions lead to attraction or to repulsion depends on the distance that separates the two groups:
** As two nonpolar groups are brought closer and closer together, the first effect is one in which a momentarily unsymmetrical distribution of electrons in one group induces an opposite polarity in the other.
** The opposite charges induced in those portions of the two groups that are in closest proximity lead to attraction between them.
** This attraction increases to a maximum as the internuclear distance of the two groups decreases.
** The internuclear distance at which the attractive force is at a maximum is equal to the sum of what are called the van der Waals radii of the two groups.
** The van der Waals radius of a group is, in effect, a measure of its size.
** If the two groups are brought still closer—closer than the sum of their van der Waals radii—their electron clouds begin to penetrate each other, and strong electron–electron repulsion occurs.
Stereoisomers and Conformational Stereoisomers
** Gauche conformers III and V of butane are examples of stereoisomers.
** Stereoisomers have the same molecular formula and connectivity but different arrangements of atoms in three-dimensional space.
** Conformational stereoisomers are related to one another by bond rotations.
** Conformational analysis is but one of the ways in which we will consider the three dimensional shapes and stereochemistry of molecules. We shall see that there are other types of stereoisomers that cannot be interconverted simply by rotations about single bonds. Among these are cis–trans cycloalkane isomers and others.
Reference: Organic chemistry / T.W. Graham Solomons , Craig B.Fryhle , Scott A.snyder , / ( eleventh edition) / 2014.
 Read Chemistry
Read Chemistry