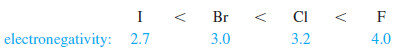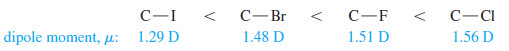Physical Properties of Alkyl Halides
Physical Properties of Alkyl Halides will be discussed such as dipole moment, London force, Dipole–dipole attractions, densities of common alkyl halides.
Structure of Alkyl Halides
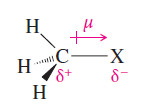
– In an alkyl halide, the halogen atom is bonded to an carbon atom.
– The halogen is more electronegative than carbon, and the bond is polarized with a partial positive charge on carbon and a partial negative charge on the halogen.
– The dipole moment (μ) is given in debyes (D):
μ = 4.8 × δ × d
where δ is the amount of charge separation, and (d) is the bond length
– The electronegativities of the halogens increase in the order
– The carbon–halogen bond lengths increase as the halogen atoms become bigger (larger atomic radii) in the order
– These two effects oppose each other, with the larger halogens having longer bonds but weaker electronegativities.
– The overall result is that the bond dipole moments increase in the order
– A molecular dipole moment is the vector sum of the individual bond dipole moments.
– Molecular dipole moments are not easy to predict because they depend on the bond angles and other factors that vary with the specific molecule.
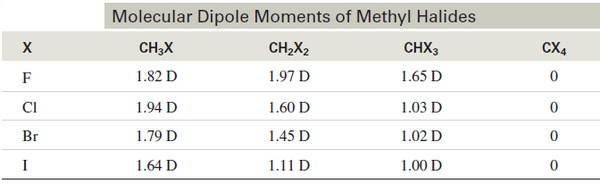
– The following table lists the experimentally measured dipole moments of the halogenated methanes.
– Notice how the four symmetrically oriented polar bonds of the carbon tetrahalides cancel to give a molecular dipole moment of zero.
Physical Properties of Alkyl Halides
– Now we will discuss Physical Properties of Alkyl Halides
– Two types of intermolecular forces influence the boiling points of alkyl halides.
– The London force is the strongest intermolecular attraction in alkyl halides.
– London forces are surface attractions, resulting from coordinated temporary dipoles.
– Molecules with larger surface areas have larger London attractions, resulting in higher boiling points.
– Dipole–dipole attractions (arising from the polar bond) also affect the boiling points, but to a smaller extent.
– Molecules with higher molecular weights generally have higher boiling points because they are heavier (and therefore slower moving), and they have greater surface area.
– The surface areas of the alkyl halides vary with the surface areas of halogens.
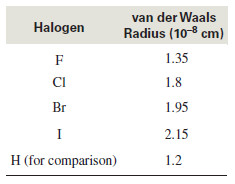
– We can get an idea of the relative surface areas of halogen atoms by considering their van der Waals radii.
– The following Figure shows that an alkyl fluoride has nearly the same surface area as the corresponding alkane; thus its London attractive forces are similar.
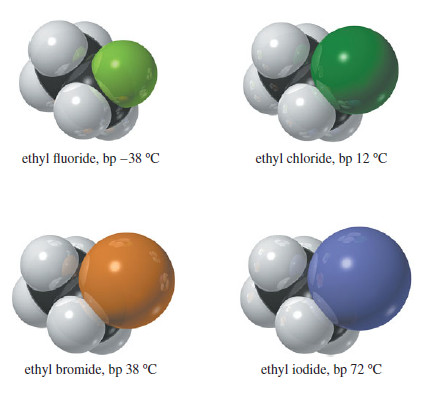
– The alkyl fluoride has a larger dipole moment, however, so the total attractive forces are slightly greater in the alkyl fluoride, giving it a higher boiling point.
– For example, the boiling point of n-butane is 0 °C, while that of n-butyl fluoride is 33 °C.
– The other halogens are considerably larger than fluorine, giving them more surface area and raising the boiling points of their alkyl halides.
– With a boiling point of 78 °C, n-butyl chloride shows the influence of chlorine’s much larger surface area.
– This trend continues with n-butyl bromide (bp 102 °C) and n-butyl iodide (bp 131 °C).
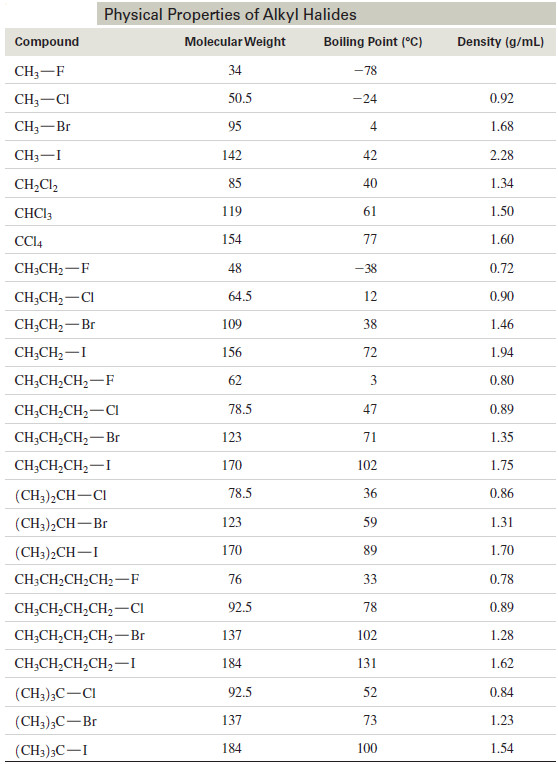
– The following Table lists the boiling points and densities of some simple alkyl halides.
– Notice that compounds with branched, more spherical shapes have lower boiling points as a result of their smaller surface areas.
– For example, n-butyl bromide has a boiling point of 102 °C, while the more spherical tert-butyl bromide has a boiling point of only 73 °C. This effect is similar to the one we saw with alkanes.
Densities of alkyl halides
– The previous Table also lists the densities of common alkyl halides.
– Like their boiling points, their densities follow a predictable trend.
– Alkyl fluorides and alkyl chlorides (those with just one chlorine atom) are less dense than water (1.00 g/mL).
– Alkyl chlorides with two or more chlorine atoms are denser than water, and all alkyl bromides and alkyl iodides are denser than water