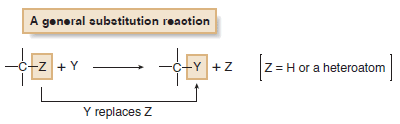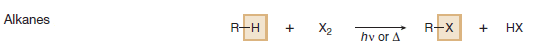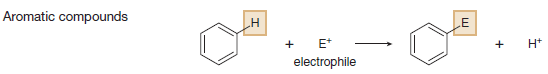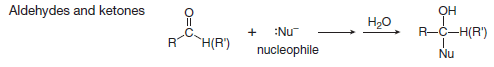Types of Organic Reactions
– In this subject, we will discuss the general Types of Organic Reactions
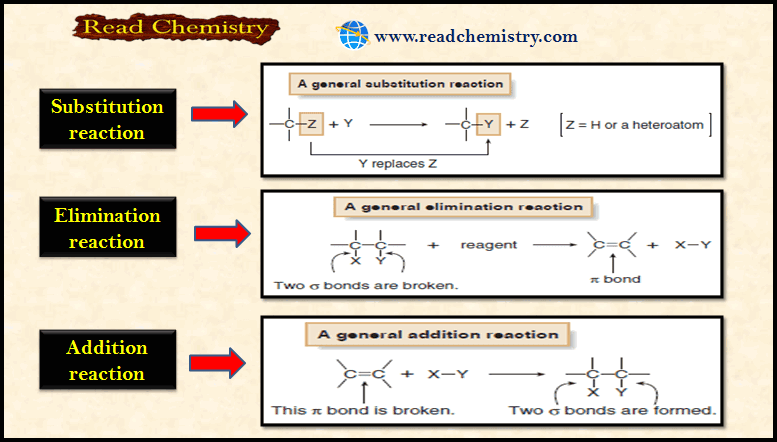
Types of Organic Reactions
– Like other compounds, organic molecules undergo acid-base and oxidation-reduction reactions.
– Organic molecules also undergo substitution, elimination, and addition reactions.
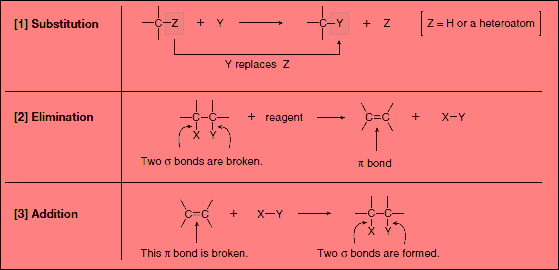
(1) Substitution Reactions
– Substitution reaction is a reaction in which an atom or a group of atoms is replaced by another atom or group of atoms.
– In a general substitution reaction, Y replaces Z on a carbon atom.
– Substitution reactions involve σ bonds: one δ bond breaks and another forms at the same carbon atom.
– The most common examples of substitution occur when Z is hydrogen or a heteroatom that is more electronegative than carbon.
Examples of Elimination Reactions
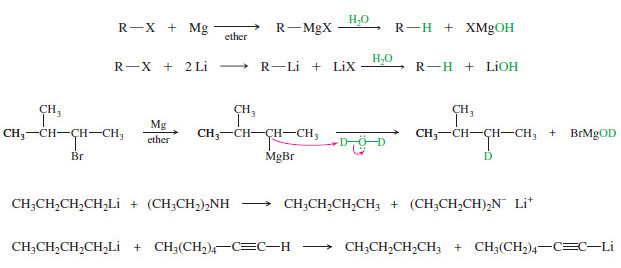
(A) Nucleophilic substitution at an sp3 hybridized carbon atom
(B) Nucleophilic acyl substitution at an sp2 hybridized carbon atom
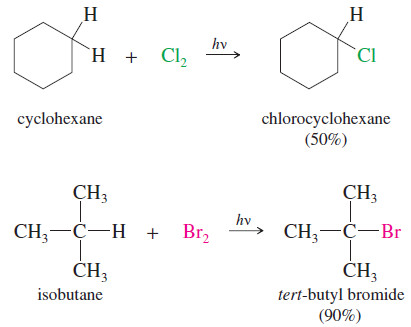
(C) Radical substitution at an sp3 hybridized C – H bond
(D) Electrophilic aromatic substitution
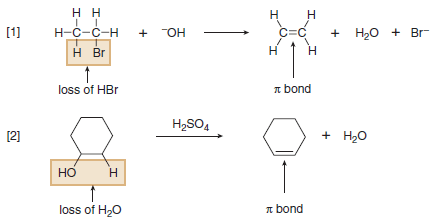
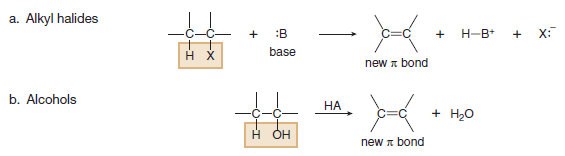
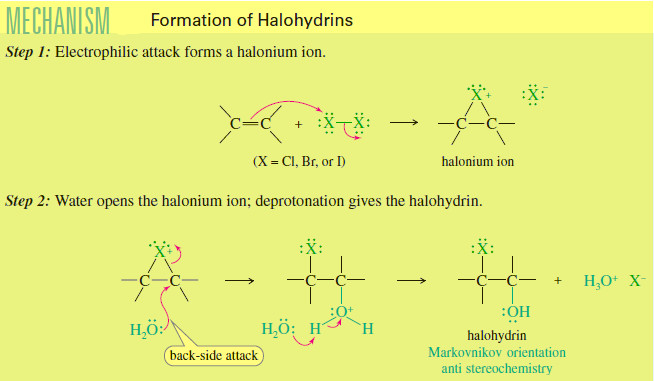
(2) Elimination Reactions
– Elimination reaction is a reaction in which elements of the starting material are (lost) and a π bond is formed.
– In an elimination reaction, two groups X and Y are removed from a starting material.
– Two σ bonds are broken, and a π bond is formed between adjacent atoms.
– The most common examples of elimination occur when X = H and Y is a heteroatom more electronegative than carbon.
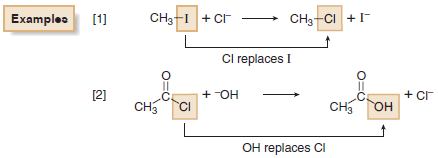
Examples of Substitution Reactions
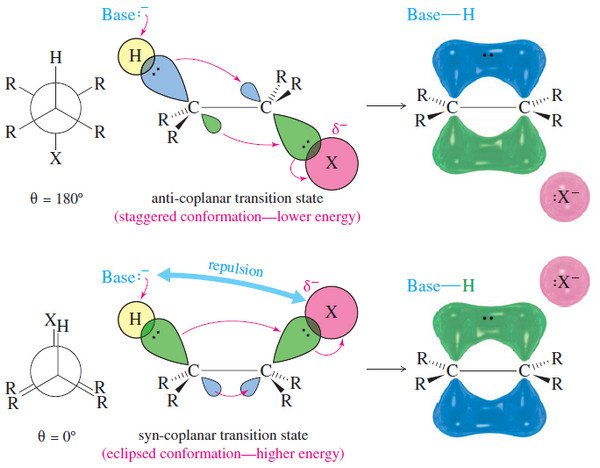
β Elimination at an sp3 hybridized carbon atom
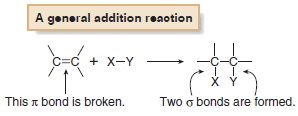
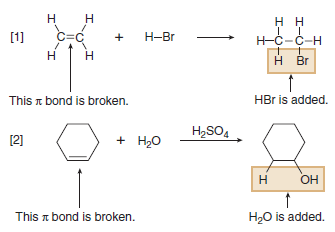
(3) Addition Reactions
– Addition reaction is a reaction in which elements are added to a starting material.
– In an addition reaction, new groups X and Y are added to a starting material.
– A π bond is broken and two σ bonds are formed.
– Addition and elimination reactions are exactly opposite.
– A π bond is formed in elimination reactions, whereas a π bond is broken in addition reactions.
Examples of Addition Reactions
(A) Electrophilic addition to carbon-carbon multiple bonds
(B) Nucleophilic addition to carbon-oxygen multiple bonds
Conclusion of general types of reaction in Organic Chemistry
Reference: Organic chemistry / Janice Gorzynski Smith, University of Hawai’i at Manoa / (Third edition), 2011. USA