Stereochemistry of the SN1 Reactions
Stereochemistry of the SN1 Reaction
– The SN2 reaction is stereospecific: the nucleophile attacks from the back side of the electrophilic carbon atom, giving inversion of configuration.
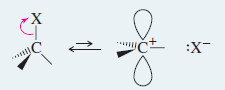
– In contrast, the SN1 reaction is not stereospecific.
– In the SN1 mechanism, the carbo-cation intermediate is sp2 hybridized and planar.
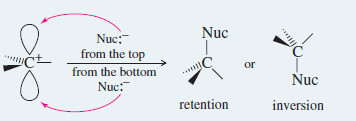
– A nucleophile can attack the carbocation from either face.
– The following Figure shows the SN1 solvolysis of a chiral compound, (S)-3-bromo-2,3-dimethylpentane, in ethanol.
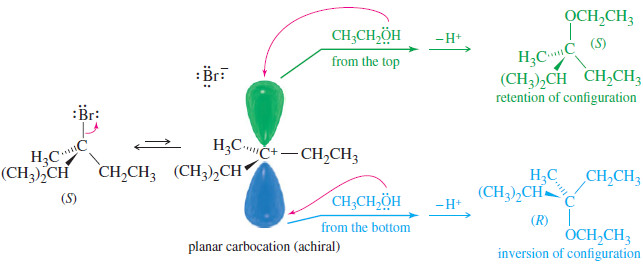
– The carbocation is planar and achiral; attack from both faces gives both enantiomers of the product.
– Such a process, giving both enantiomers of the product (whether or not the two enantiomers are produced in equal amounts), is called racemization.
– The product is either racemic or at least less optically pure than the starting material.
– If a nucleophile attacks the carbocation in the previous Figure from the front side (the side the leaving group left), the product molecule shows retention of configuration.
– Attack from the back side gives a product molecule showing inversion of configuration.
– Racemization is simply a combination of retention and inversion.
– When racemization occurs, the product is rarely completely racemic, however; there is often more inversion than retention of configuration.
– As the leaving group leaves, it partially blocks the front side of the carbocation.
– The back side is unhindered, so attack is more likely there.
– The following Figure shows a cyclic case where one of the faces of a cyclopentane ring has been (labeled) by a deuterium atom.
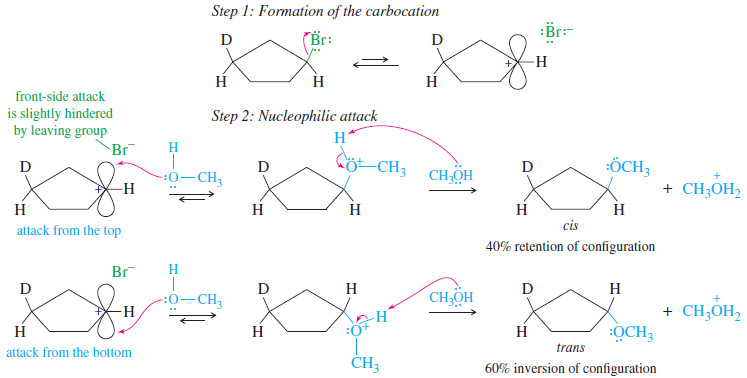
– Deuterium has the same size and shape as hydrogen and it undergoes the same reactions.
– It distinguishes between the two faces of the ring: the bromine atom is cis to the deuterium in the reactant, so the nucleophile is cis to the deuterium in the retention product.
– The nucleophile is trans to the deuterium in the inversion product.
– The product mixture contains both cis and trans isomers, with the trans isomer slightly favored because the leaving group hinders approach of the nucleophilic solvent from the front side.
MECHANISM: Racemization in the SN1 Reaction
The SN1 reaction involves ionization to a flat carbocation, which can be attacked from either side.
Step 1: Ionization of a tetrahedral carbon gives a flat carbocation
Step 2: A nucleophile may attack either side of the carbocation.
These two products may be different if the carbon atom is stereogenic.