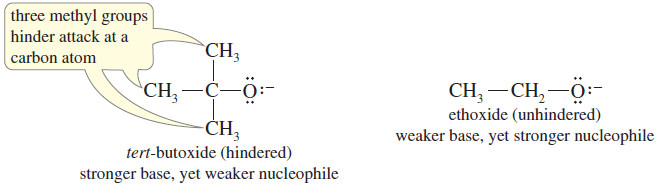Factors Affecting SN2 Reactions: Strength of the Nucleophile
Factors Affecting SN2 Reactions: Strength of the Nucleophile
– we will discuss Factors Affecting SN2 Reactions especially Strength of the Nucleophile
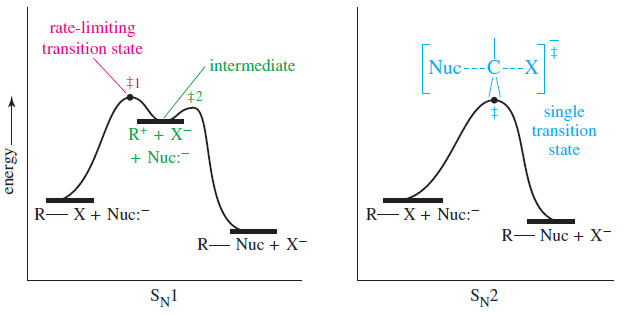
– We will use the SN2 reaction as an example of how we study the properties of the species that participate in the reaction.
– Both the nucleophile and the substrate (the alkyl halide) are important, as well as the type of solvent used.
– We begin by considering what makes a good nucleophile.
– A “stronger” nucleophile is an ion or molecule that reacts faster in the SN2 reaction than a “weaker” nucleophile under the same conditions.
– A strong nucleophile is much more effective than a weak one in attacking an electrophilic carbon atom.
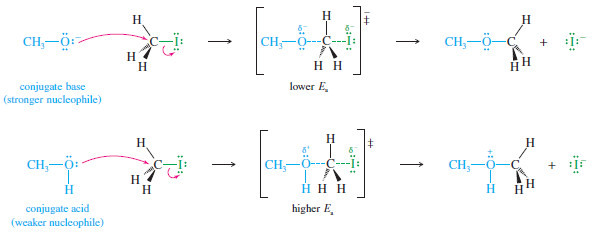
– For example, both methanol (CH3OH) and methoxide ion (CH3O–) have easily shared pairs of nonbonding electrons, but methoxide ion reacts with electrophiles in the SN2 reaction about 1 million times faster than methanol.
– It is generally true that a species with a negative charge is a stronger nucleophile than a similar, neutral species.
– Methoxide ion has nonbonding electrons that are readily available for bonding.
– In the transition state, the negative charge is shared by the oxygen of methoxide ion and by the halide leaving group.
– Methanol, however, has no negative charge; the transition state has a partial negative charge on the halide but a partial positive charge on the methanol oxygen atom.
– We can generalize the case of methanol and the methoxide ion to say that:
A base is always a stronger nucleophile than its conjugate acid.
– We might be tempted to say that methoxide is a much better nucleophile because it is much more basic.
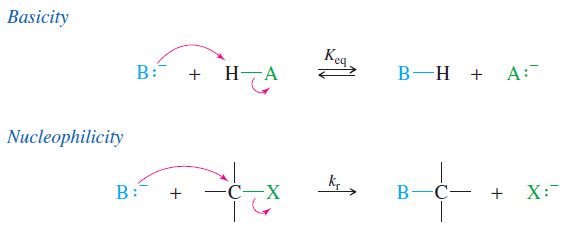
– This would be a mistake because basicity and nucleophilicity are different properties.
– Basicity is defined by the equilibrium constant for abstracting a proton.
– Nucleophilicity is defined by the rate of attack on an electrophilic carbon atom. In both cases, the nucleophile (or base) forms a new bond.
– If the new bond is to a proton, it has reacted as a base; if the new bond is to carbon, it has reacted as a nucleophile.
– Predicting which way a species will react may be difficult; most (but not all) good nucleophiles are also strong bases, and vice versa.
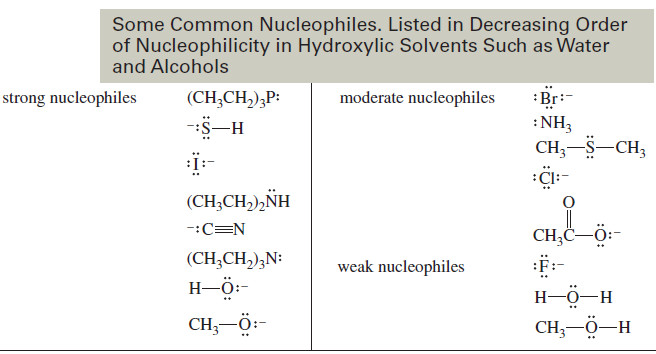
– The following table lists some common nucleophiles in decreasing order of their nucleophilicity in hydroxylic solvents such as water and alcohols.
– The strength of nucleophiles in these solvents shows three major trends:
SUMMARY Trends in Nucleophilicity (Nucleophile)
1. A species with a negative charge is a stronger nucleophile than a similar neutral species. In particular, a base is a stronger nucleophile than its conjugate acid
2. Nucleophilicity decreases from left to right in the periodic table, following the increase in electronegativity from left to right. The more electronegative elements have more tightly held nonbonding electrons that are less reactive toward forming new bonds.
3. Nucleophilicity increases down the periodic table, following the increase in size and polarizability, and the decrease in electronegativity.
– The third trend (size and polarizability) reflects an atom’s ability to engage in partial bonding as it begins to attack an electrophilic carbon atom.
– As we go down a column in the periodic table, the atoms become larger, with more electrons at a greater distance from the nucleus.
– The electrons are more loosely held, and the atom is more polarizable: Its electrons can move more freely toward a positive charge, resulting in stronger bonding in the transition state.
– The increased mobility of its electrons enhances the atom’s ability to begin to form a bond at a relatively long distance.
– the following Figure illustrates this polarizability effect by comparing the attack of iodide ion and fluoride ion on a methyl halide.
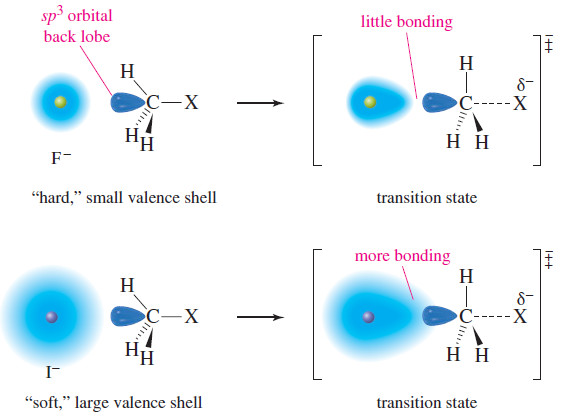
– The outer shell of the fluoride ion is the second shell.
– These electrons are tightly held, close to the nucleus.
– Fluoride is a “hard” (low-polarizability) nucleophile, and its nucleus must approach the carbon nucleus quite closely before the electrons can begin to overlap and form a bond.
– In the transition state, there is little bonding between fluorine and carbon. In contrast, the outer shell of the iodide ion is the fifth shell.
– These electrons are loosely held, making the iodide ion a “soft” (high-polarizability) nucleophile.
– The outer electrons begin to shift and overlap with the carbon atom from farther away.
– There is a great deal of bonding between iodine and carbon in the transition state, which lowers the energy of the transition state.
Steric Effects on Nucleophilicity (Nucleophile)
– To serve as a nucleophile, an ion or molecule must get in close to a carbon atom to attack it.
– Bulky groups on the nucleophile hinder this close approach, and they slow the reaction rate.
– For example, the tert butoxide ion is a stronger base (for abstracting protons) than ethoxide ion, but tert-butoxide ion has three methyl groups that hinder any close approach to a more crowded carbon atom.
– Therefore, ethoxide ion is a stronger nucleophile than tert-butoxide ion.
– When bulky groups interfere with a reaction by virtue of their size, we call the effect steric hindrance.
– Steric hindrance has little effect on basicity because basicity involves attack on an unhindered proton.
– When a nucleophile attacks a carbon atom, however, a bulky nucleophile cannot approach the carbon atom so easily.
– Most bases are also nucleophiles, capable of attacking either a proton or an electrophilic carbon atom.
– If we want a species to act as a base, we use a bulky reagent like tert-butoxide ion.
– If we want it to react as a nucleophile, we use a less hindered reagent, like ethoxide.
Solvent Effects on Nucleophilicity
– Another factor affecting the nucleophilicity of these ions is their solvation, particularly in protic solvents.
– A protic solvent is one that has acidic protons, usually in the form of O-H or N-H groups.
– These groups form hydrogen bonds to negatively charged nucleophiles.
– Protic solvents, especially alcohols, are convenient solvents for nucleophilic substitutions because the reagents (alkyl halides, nucleophiles, etc.) tend to be quite soluble.
– Small anions are solvated more strongly than large anions in a protic solvent because the solvent approaches a small anion more closely and forms stronger hydrogen bonds.
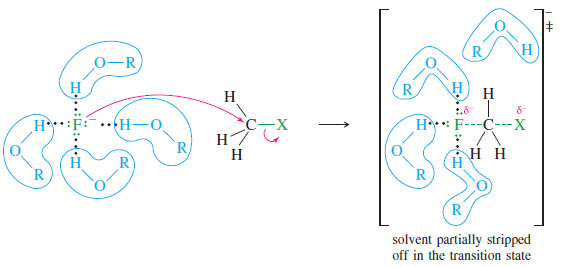
– When an anion reacts as a nucleophile, energy is required to “strip off” some of the solvent molecules, breaking some of the hydrogen bonds that stabilized the solvated anion.
– More energy is required to strip off solvent from a small, strongly solvated ion such as fluoride than from a large, diffuse, less strongly solvated ion like iodide.
– The enhanced solvation of smaller anions in protic solvents, requiring more energy to strip off their solvent molecules, reduces their nucleophilicity.
– This trend reinforces the trend in polarizability: The polarizability increases with increasing atomic number, and the solvation energy (in protic solvents) decreases with increasing atomic number.
– Therefore, nucleophilicity (in protic solvents) generally increases down a column in the periodic table, as long as we compare similar species with similar charges.
– In contrast with protic solvents, aprotic solvents (solvents without O-H or N-H groups) enhance the nucleophilicity of anions.
– An anion is more reactive in an aprotic solvent because it is not so strongly solvated.
– There are no hydrogen bonds to be broken when solvent must make way for the nucleophile to approach an electrophilic carbon atom.
– The relatively weak solvating ability of aprotic solvents is also a disadvantage: Most polar, ionic reagents are insoluble in simple aprotic solvents such as alkanes.
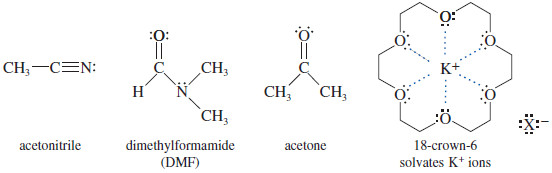
Polar aprotic solvents
– Polar aprotic solvents have strong dipole moments to enhance solubility, yet they have no O-H or N-H groups to form hydrogen bonds with anions.
– Examples of useful polar aprotic solvents are acetonitrile, dimethylformamide, and acetone.
– We can add specific solvating reagents to enhance solubility without affecting the reactivity of the nucleophile.
– For example, the “crown ether” 18 crown-6 solvates potassium ions.
– Using the potassium salt of a nucleophile and solvating the potassium ions causes the nucleophilic anion to be dragged along into solution.
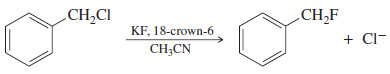
– The following example shows how fluoride ion, normally a poor nucleophile in hydroxylic (protic) solvents, can be a good nucleophile in an aprotic solvent.
– Although KF is not very soluble in acetonitrile, 18-crown-6 solvates the potassium ions, and the poorly solvated (and therefore nucleophilic) fluoride ion follows.