Stereochemistry of the E2 Reaction
Stereochemistry of the E2 Reaction
– In this subject Stereochemistry of the E2 Reaction will be discussed
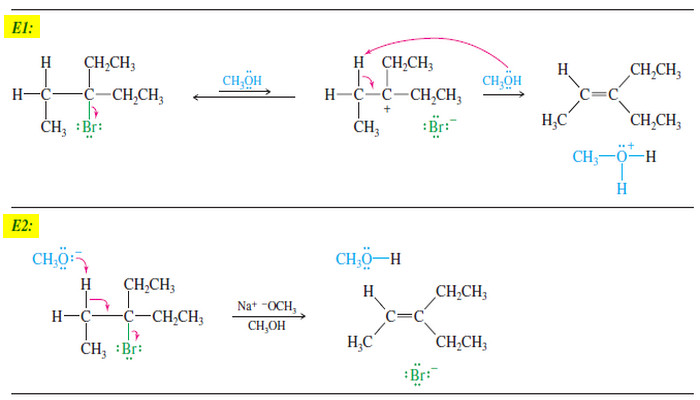
– Like the SN2 reaction, the E2 reaction follows a concerted mechanism: Bond breaking and bond formation take place at the same time, and the partial formation of new bonds lowers the energy of the transition state.
– Concerted mechanisms require specific geometric arrangements so that the orbitals of the bonds being broken can overlap with those being formed and the electrons can flow smoothly from one bond to another.
– The geometric arrangement required by the SN2 reaction is a back-side attack; with the E2 reaction, a coplanar arrangement of the orbitals is needed.
– E2 elimination requires partial formation of a new pi bond, with its parallel p orbitals, in the transition state.
– The electrons that once formed a C-H bond must begin to overlap with the orbital that the leaving group is vacating.
– Formation of this new pi bond implies that these two sp3 orbitals must be parallel so that pi overlap is possible as the hydrogen and halogen leave and the orbitals rehybridize to the p orbitals of the new pi bond.
– The following Figure shows two conformations that provide the necessary coplanar alignment of the leaving group, the departing hydrogen, and the two carbon atoms.
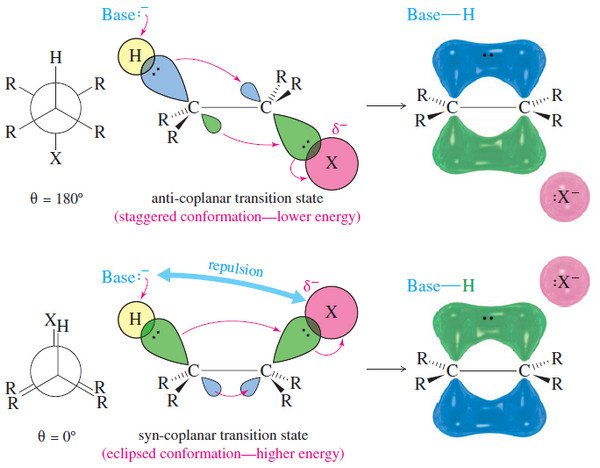
– When the hydrogen and the halogen are anti to each other (θ = 180°) their orbitals are aligned. This is called the anti-coplanar conformation.
– When the hydrogen and the halogen eclipse each other (θ = 0°) their orbitals are once again aligned. This is called the syn-coplanar conformation.
– Make a model corresponding to Figure above, and use it to follow along with this discussion.
– Of these possible conformations, the anti-coplanar arrangement is most commonly seen in E2 reactions.
– The transition state for the anti-coplanar arrangement is a staggered conformation, with the base far away from the leaving group.
– In most cases, this transition state is lower in energy than that for the syn-coplanar elimination.
– The transition state for syn-coplanar elimination is an eclipsed conformation.
– In addition to the higher energy resulting from eclipsing interactions, the transition state suffers from interference between the attacking base and the leaving group.
– To abstract the proton, the base must approach quite close to the leaving group.
– In most cases, the leaving group is bulky and negatively charged, and the repulsion between the base and the leaving group raises the energy of the syn-coplanar transition state.
– Some molecules are rigidly held in eclipsed (or nearly eclipsed) conformations, with a hydrogen atom and a leaving group in a syn coplanar arrangement.
– Such compounds are likely to undergo E2 elimination by a concerted syn-coplanar mechanism.
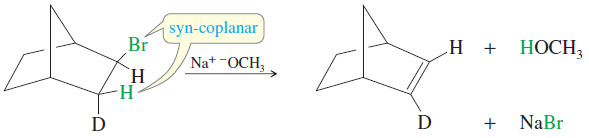
– Deuterium labeling (using D, the hydrogen isotope with mass number 2) is used in the following reaction to show which atom is abstracted by the base.
– Only the hydrogen atom is abstracted, because it is held in a syn-coplanar position with the bromine atom.
– Remember that syn-coplanar eliminations are unusual, however, and anti-coplanar eliminations are more common.
– The E2 is a stereospecific reaction, because different stereoisomers of the starting material react to give different stereoisomers of the product.
– This stereospecificity results from the anti-coplanar transition state that is usually involved in the E2.