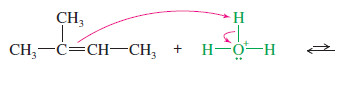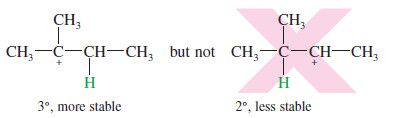Hydration of Alkenes: Addition of Water
Hydration of Alkenes will be discussed by Addition of Water
Addition of Water: Hydration of Alkenes
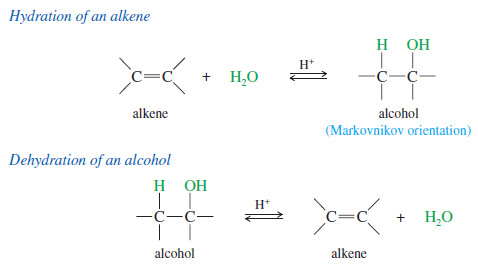
– An alkene may react with water in the presence of a strongly acidic catalyst to form an alcohol.
– Formally, this reaction is a hydration (the addition of water), with a hydrogen atom adding to one carbon and a hydroxyl group adding to the other.
– Hydration of alkenes are the reverse of the dehydration of alcohols.
– For dehydrating alcohols, a concentrated dehydrating acid (such as H2SO4 or H3PO4) is used to drive the equilibrium to favor the alkene.
– Hydration of an alkene, on the other hand, is accomplished by adding excess water to drive the equilibrium toward the alcohol
(A) Mechanism of Hydration of Alkenes
– The principle of microscopic reversibility states that a forward reaction and a reverse reaction taking place under the same conditions (as in an equilibrium) must follow the same reaction pathway in microscopic detail.
– The hydration and dehydration reactions are the two complementary reactions in an equilibrium; therefore, they must follow the same reaction pathway.
– It makes sense that the lowest-energy transition states and intermediates for the reverse reaction are the same as those for the forward reaction, except in reverse order.
– According to the principle of microscopic reversibility, we can write the hydration mechanism by reversing the order of the steps of the dehydration .
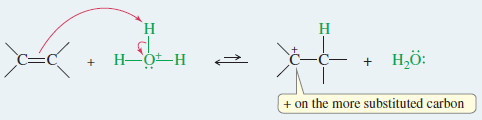
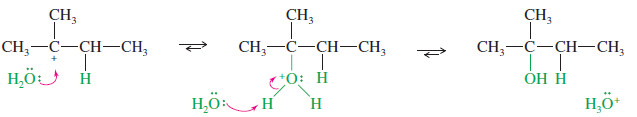
– Protonation of the double bond forms a carbocation.
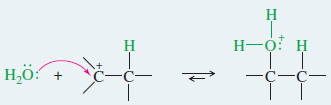
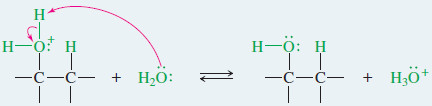
– Nucleophilic attack by water, followed by loss of a proton, gives the alcohol.
MECHANISM: Acid-Catalyzed Hydration of an Alkene
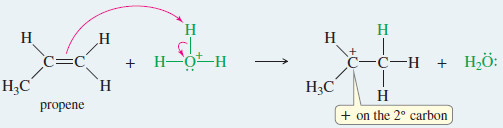
Step 1: Protonation of the double bond forms a carbocation.
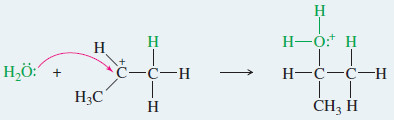
Step2: Nucleophilic attack by water gives a protonated alcohol.
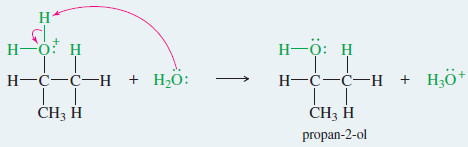
Step 3: Deprotonation gives the alcohol.
EXAMPLE: Acid-catalyzed hydration of propene.
Step 1: Protonation of the double bond forms a secondary carbocation.
Step2: Nucleophilic attack by water gives a protonated alcohol.
Step 3: Deprotonation gives the alcohol.
(B) Orientation of Hydration
– Step (1) of the hydration mechanism is similar to the first step in the addition of HBr.
– The proton adds to the less substituted end of the double bond to form the more substituted carbocation.
– Water attacks the carbocation to give (after loss of a proton) the alcohol with the -OH group on the more substituted carbon.
– Like the addition of hydrogen halides, hydration is regioselective: It follows Markovnikov’s rule, giving a product in which the new hydrogen has added to the less substituted end of the double bond. Consider the hydration of 2-methylbut-2-ene:
– The proton adds to the less substituted end of the double bond, so the positive charge appears at the more substituted end.
– Water attacks the carbocation to give the protonated alcohol.
– The reaction follows Markovnikov’s rule. The proton has added to the end of the double bond that already had more hydrogens (that is, the less substituted end), and the group has added to the more substituted end.
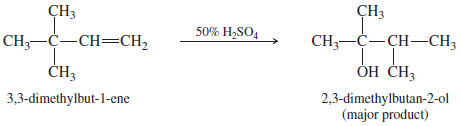
– Like other reactions that involve carbocation intermediates, hydration may take place with rearrangement.
– For example, when 3,3-dimethylbut-1-ene undergoes acidcatalyzed hydration, the major product results from rearrangement of the carbocation intermediate.