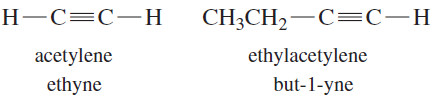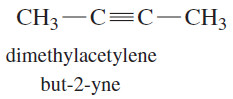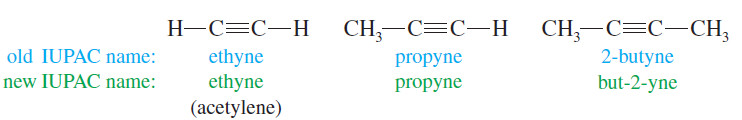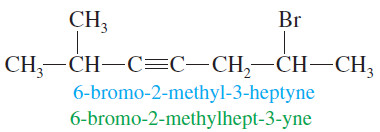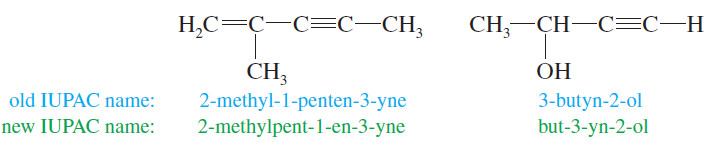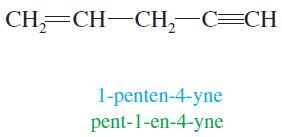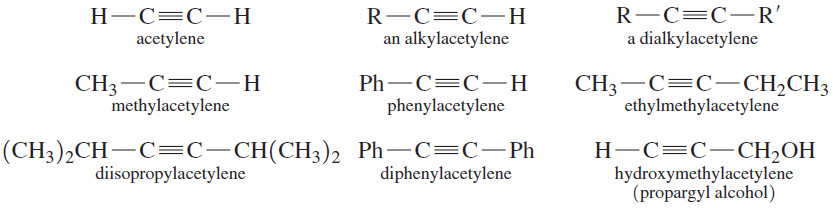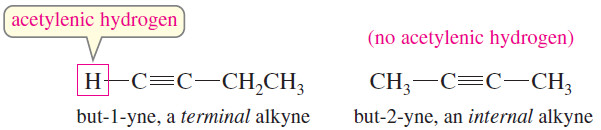Nomenclature of Alkynes
What are Alkynes?
– Alkynes are hydrocarbons that contain carbon–carbon triple bonds.
– Alkynes are also called acetylenes because they are derivatives of acetylene, the simplest alkyne
– The chemistry of the carbon–carbon triple bond is similar to that of the double bond.
– Alkynes undergo most of the same reactions as alkenes, especially the additions and the oxidations.
– Reactions that are specific to alkynes: some that depend on the unique characteristics of the C ≡ C triple bond, and others that depend on the unusual acidity of the acetylenic ≡ C – H bond.
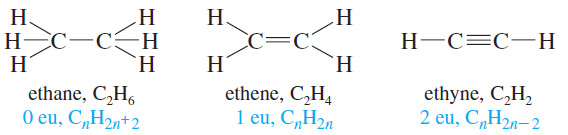
– A triple bond gives an alkyne four fewer hydrogens than the corresponding alkane.
– Its molecular formula is like that of a molecule with two double bonds: CnH2n-2 Therefore, the triple bond contributes two elements of unsaturation (eu).
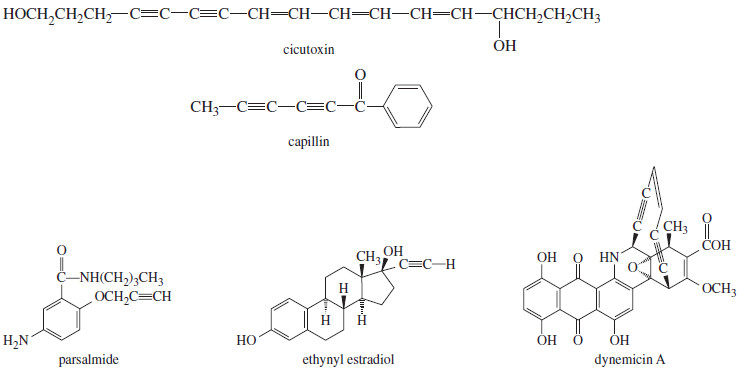
– Alkynes are not as common in nature as alkenes, but some plants do use alkynes to protect themselves against disease or predators.
– Cicutoxin is a toxic compound found in water hemlock, and capillin protects a plant against fungal diseases.
– The alkyne functional group is uncommon in drugs, but parsalmide is used as an analgesic, and ethynyl estradiol (a synthetic female hormone) is a common ingredient in birth control pills.
– Dynemicin A is an antibacterial compound that is being tested as an antitumor agent
Nomenclature of Alkynes
IUPAC Names
– The IUPAC nomenclature for alkynes is similar to that for alkenes.
– We find the longest continuous chain of carbon atoms that includes the triple bond and change the -ane ending of the parent alkane to –yne.
– The chain is numbered from the end closest to the triple bond, and the position of the triple bond is designated by its lowernumbered carbon atom.
– Substituents are given numbers to indicate their locations
– When additional functional groups are present, the suffixes are combined to produce the compound names of the alkenynes (a double bond and a triple bond), alkynols (a triple bond and an alcohol), and so on.
– The new IUPAC system (placing the number right before the group) helps to clarify these names.
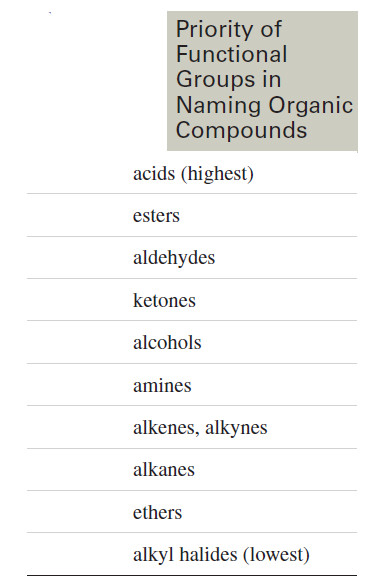
– The IUPAC rules give alcohols higher priority than alkenes or alkynes (which are given equal priority), so the numbering begins at the end closer to an alcohol.
– The priorities of functional groups in naming organic compounds are listed in the following table.
– If the double bond and the triple bond are equidistant from the ends of the chain, number the chain so that the double bond receives a lower number than the triple bond (because “ene” comes before “yne” in the alphabet).
Common Names
– The common names of alkynes describe them as derivatives of acetylene.
– Most alkynes can be named as a molecule of acetylene with one or two alkyl substituents.
– This nomenclature is like the common nomenclature for ethers, where we name the two alkyl groups bonded to oxygen.
– Many of an alkyne’s chemical properties depend on whether there is an acetylenic hydrogen (H – C ≡ C) that is, whether the triple bond comes at the end of a carbon chain.
– Such an alkyne is called a terminal alkyne or a terminal acetylene.
– If the triple bond is located somewhere other than the end of the carbon chain, the alkyne is called an internal alkyne or an internal acetylene.