Physical Properties of Alkenes
Physical Properties of Alkenes
(1) Boiling Points and Densities
– Most physical properties of alkenes are similar to those of the corresponding alkanes.
– For example, the boiling points of but-1-ene, cis-but-2-ene, trans-but-2-ene, and n-butane are all close to 0 °C.
– Also like the alkanes, alkenes have densities around 0.6 or 0.7g/cm3
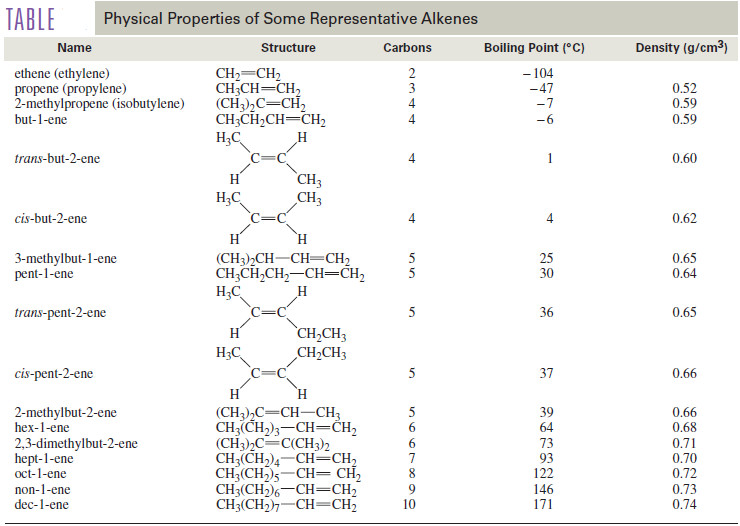
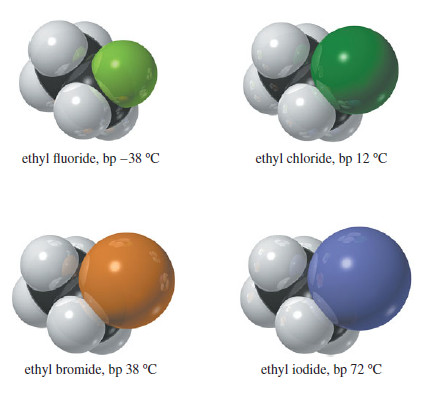
– The boiling points and densities of some representative alkenes are listed in the following Table.
– The table shows that boiling points of alkenes increase smoothly with molecular weight.
– As with alkanes, increased branching leads to greater volatility and lower boiling points.
– For example, 2-methylpropene (isobutylene) has a boiling point of which is lower than the boiling point of any of the unbranched butenes.
(2) Polarity
– Like alkanes, alkenes are relatively nonpolar.
– They are insoluble in water but soluble in nonpolar solvents such as hexane, gasoline, halogenated solvents, and ethers.
– Alkenes tend to be slightly more polar than alkanes, however, for two reasons: The more weakly held electrons in the pi bond are more polarizable (contributing to instantaneous dipole moments), and the vinylic bonds tend to be slightly polar (contributing to a permanent dipole moment).
– Alkyl groups are slightly electron-donating toward a double bond, helping to stabilize it.
– This donation slightly polarizes the vinylic bond, with a small partial positive charge on the alkyl group and a small negative charge on the double-bond carbon atom.
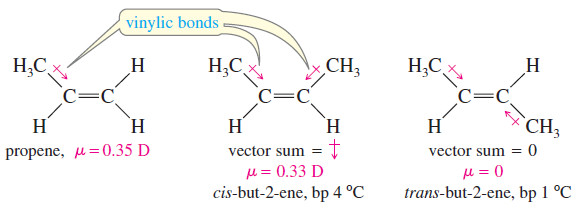
– For example, propene has a small dipole moment of 0.35 D.
– In a cis-disubstituted alkene, the vector sum of the two dipole moments is directed perpendicular to the double bond.
– In a trans-disubstituted alkene, the two dipole moments tend to cancel out.
– If an alkene is symmetrically trans disubstituted, the dipole moment is zero.
– For example, cis-but-2-ene has a nonzero dipole moment, but trans-but-2-ene has no measurable dipole moment.
– Compounds with permanent dipole moments engage in dipole–dipole attractions, while those without permanent dipole moments engage only in van der Waals attractions.
– cis-But-2-ene and trans-but-2-ene have similar van der Waals attractions, but only the cis isomer has dipole dipole attractions.
– Because of its increased intermolecular attractions, cis-but-2-ene must be heated to a slightly higher temperature (4 °C versus 1 °C) before it begins to boil.
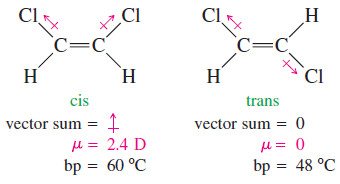
– The effect of bond polarity is even more apparent in the 1,2-dichloroethenes, with their strongly polar carbon–chlorine bonds.
– The cis isomer has a large dipole moment (2.4 D), giving it a boiling point 12 degrees higher than the trans isomer, with zero dipole moment.