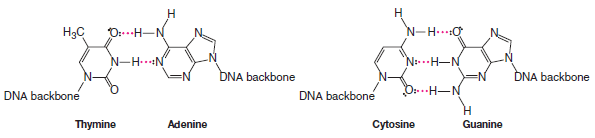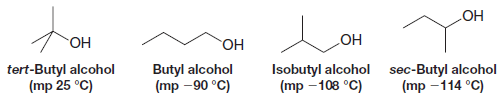Intermolecular Forces in Organic compounds
– In this subject, we will discuss the Intermolecular Forces in Organic compounds
Intermolecular Forces in Organic Compounds
– The forces that act between molecules are not as strong as those between ions, but they account for the fact that even completely nonpolar molecules can exist in liquid and solid states.
– These intermolecular forces, collectively called van der Waals forces, are all electrical.
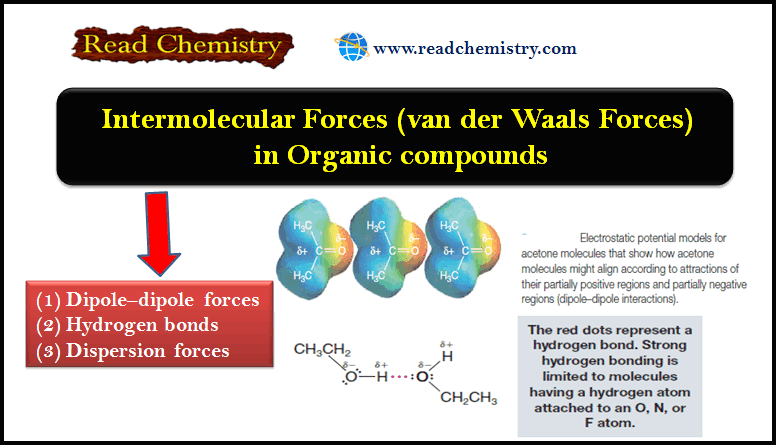
– We will focus our attention on three types of Intermolecular Forces in Organic compounds:
- Dipole–dipole forces
- Hydrogen bonds
- Dispersion forces
(A) Dipole-Dipole Forces
– Most organic molecules are not fully ionic but have instead a permanent dipole moment resulting from a nonuniform distribution of the bonding electrons.
– Acetone and acetaldehyde are examples of molecules with permanent dipoles because the carbonyl group that they contain is highly polarized.
– In these compounds, the attractive forces between molecules are much easier to visualize.
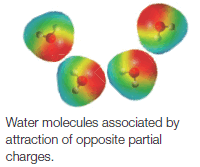
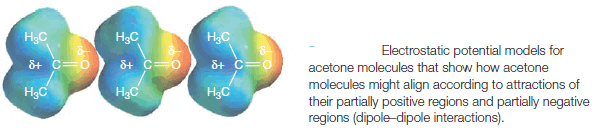
– In the liquid or solid state, dipole-dipole attractions cause the molecules to orient themselves so that the positive end of one molecule is directed toward the negative end of another (Fig. 1).
(B) Hydrogen Bonds
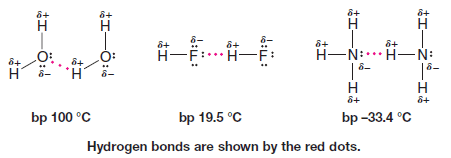
– Very strong dipole-dipole attractions occur between hydrogen atoms bonded to small, strongly electronegative atoms (O, N, or F) and nonbonding electron pairs on other such electronegative atoms.
– This type of intermolecular force is called a hydrogen bond.
– Hydrogen bonds (bond dissociation energies of about 4 – 38 kJ mol-1) are weaker than ordinary covalent bonds but much stronger than the dipole-dipole interactions that occur above, for example, in acetone.
– Hydrogen bonding explains why water, ammonia, and hydrogen fluoride all have far higher boiling points than methane (bp -161.6 oC), even though all four compounds have similar molecular weights.
– One of the most important consequences of hydrogen bonding is that it causes water to be a liquid rather than a gas at 25 oC.
– Calculations indicate that in the absence of hydrogen bonding, water would have a boiling point near -80 oC and would not exist as a liquid unless the temperature were lower than that temperature.
– Had this been the case, it is highly unlikely that life, as we know it, could have developed on the planet Earth.
– Hydrogen bonds hold the base pairs of double-stranded DNA together.
– Thymine hydrogen bonds with adenine. Cytosine hydrogen bonds with guanine.
– Hydrogen bonding accounts for the fact that ethyl alcohol has a much higher boiling point (78.5 oC) than dimethyl ether (24.9 oC) even though the two compounds have the same molecular weight.
– Molecules of ethyl alcohol, because they have a hydrogen atom covalently bonded to an oxygen atom, can form strong hydrogen bonds with each other.
– Molecules of dimethyl ether, because they lack a hydrogen atom attached to a strongly electronegative atom, cannot form strong hydrogen bonds with each other.
– In dimethyl ether, the intermolecular forces are weaker dipole-dipole interactions
– A factor (in addition to polarity and hydrogen bonding) that affects the melting point of many organic compounds is the compactness and rigidity of their individual molecules.
– Symmetrical molecules generally have abnormally high melting points.
– Tert-butyl alcohol, for example, has a much higher melting point than the other isomeric alcohols shown here:
(C) Dispersion Forces
If we consider a substance like methane where the particles are nonpolar molecules, we find that the melting point and boiling point are very low: -182.6 oC and -162 oC, respectively.
Instead of asking, “Why does methane melt and boil at low temperatures?” a more appropriate question might be “Why does methane, a nonionic, nonpolar substance, become a liquid or a solid at all?” The answer to this question can be given in terms of attractive intermolecular forces called dispersion forces or London forces.
An accurate account of the nature of dispersion forces requires the use of quantum mechanics.
We can, however, visualize the origin of these forces in the following way:
(1) The average distribution of charge in a nonpolar molecule (such as methane) over some time is uniform.
(2) At any given instant, however, because electrons move, the electrons and therefore the charge may not be uniformly distributed.
(3) Electrons may, in one instant, be slightly accumulated on one part of the molecule, and, as a consequence, a small temporary dipole will occur.
(4) This temporary dipole in one molecule can induce opposite (attractive) dipoles in surrounding molecules.
– It does this because the negative (or positive) charge in a portion of one molecule will distort the electron cloud of an adjacent portion of another molecule, causing an opposite charge to develop there.
(5) These temporary dipoles change constantly, but the net result of their existence is to produce attractive forces between nonpolar molecules and thus make possible the existence of their liquid and solid states.
– Two important factors determine the magnitude of dispersion forces.
(1) The relative polarizability of electrons of the atoms involved.
– By polarizability, we mean how easily the electrons respond to a changing electric field.
– The electrons of large atoms such as iodine are loosely held and are easily polarized, while the electrons of small atoms such as fluorine are more tightly held and are much less polarizable.
– CF4 and CI4 are both nonpolar molecules.
– But if we were to consider the intermolecular forces between two CI4 molecules, which contain polarizable iodine atoms, we would find that the dispersion forces are much larger than between two CF4 molecules, which contain fluorine atoms that are not very polarizable.
(2) The relative surface area of the molecules involved.
– The larger the surface area, the larger the overall attraction between molecules caused by dispersion forces.
– Molecules that are generally longer, flatter, or cylindrical have a greater surface area available for intermolecular interactions than more spherical molecules and consequently have greater attractive forces between them than the tangential interactions between branched molecules.
– This is evident when comparing pentane, the unbranched C5H12 hydrocarbon, with neopentane, the most highly branched C5H12 isomer (in which one carbon bears four methyl groups).
– Pentane has a boiling point of 36.1 oC. Neopentane has a boiling point of 9.5 oC.
– The difference in their boiling points indicates that the attractive forces between pentane molecules are stronger than between neopentane molecules.
– For large molecules, the cumulative effect of these small and rapidly changing dispersion forces can lead to a large net attraction.
Intermolecular Forces in Biochemistry
– Later, after we have had a chance to examine in detail the properties of the molecules that make up living organisms, we shall see how intermolecular forces are extremely important in the functioning of cells.
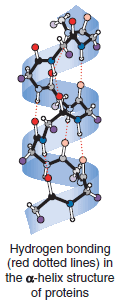
– Hydrogen bond formation, the hydration of polar groups, and the tendency of nonpolar groups to avoid a polar environment all cause complex protein molecules to fold in precise ways—ways that allow them to function as biological catalysts of incredible efficiency.
– The same factors (above) allow molecules of hemoglobin to assume the shape needed to transport oxygen.
– They allow proteins and molecules called lipids to function as cell membranes.
– Hydrogen bonding gives certain carbohydrates a globular shape that makes them highly efficient food reserves in animals.
– It gives molecules of other carbohydrates a rigid linear shape that makes them perfectly suited to be structural components in plants.
Reference: Organic chemistry / T.W. Graham Solomons, Craig B.Fryhle, Scott A. Snyder, / ( eleventh edition) / 2014.