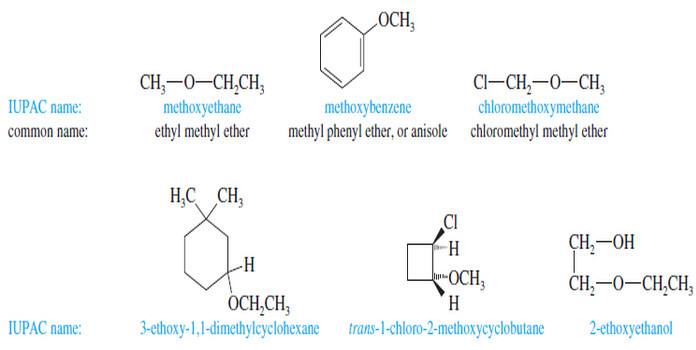
Characteristic Absorptions of Alcohols and Amines
– In this topic, we will discuss the Characteristic Absorptions of Alcohols and Amines by examples.
Characteristic Absorptions of Alcohols and Amines
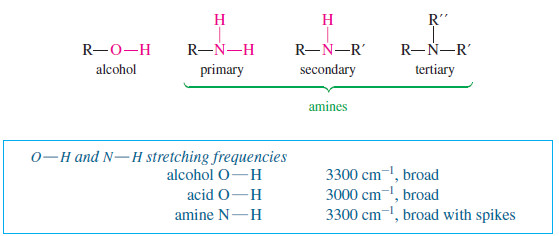
– The O-H bonds of alcohols and the N-H bonds of amines are strong and stiff.
– The vibration frequencies of O-H and N-H bonds therefore occur at higher frequencies than those of most C-H bonds (except for alkynyl ≡C-H bonds).
Characteristic Absorptions of Alcohols
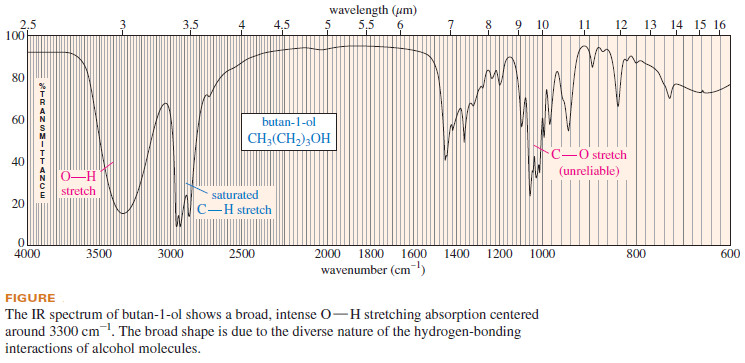
– Alcohol O-H bonds absorb over a wide range of frequencies, centered around 3300 cm-1
– Alcohol molecules are involved in hydrogen bonding, with different molecules having different instantaneous arrangements.
– The O-H stretching frequencies reflect this diversity of hydrogen-bonding arrangements, resulting in very broad absorptions.
– Notice the broad O-H absorption centered around 3300 cm-1 in the infrared spectrum of butan-1-ol (see Figure).
– Like alcohols, carboxylic acids give O-H absorptions that are broadened by hydrogen bonding.
– The broad acid O-H absorption is usually centered around 3000 cm-1, however (compared with 3300 cm-1 for an alcohol), because of the stronger hydrogen bonding between acid molecules.
– The previous Figure also shows a strong C-O stretching absorption centered near 1060 cm-1
– Compounds with C-O bonds (alcohols and ethers, for example) generally show strong absorptions in the range of 1000 to 1200 cm-1 however, there are other functional groups that also absorb in this region.
– Therefore, a strong peak between 1000 and 1200 cm-1 does not necessarily imply a C-O bond, but the absence of an absorption in this region suggests the absence of a C-O bond. For simple ethers, this unreliable C-O absorption is usually the only clue that the compound might be an ether.
Absorptions of Amines
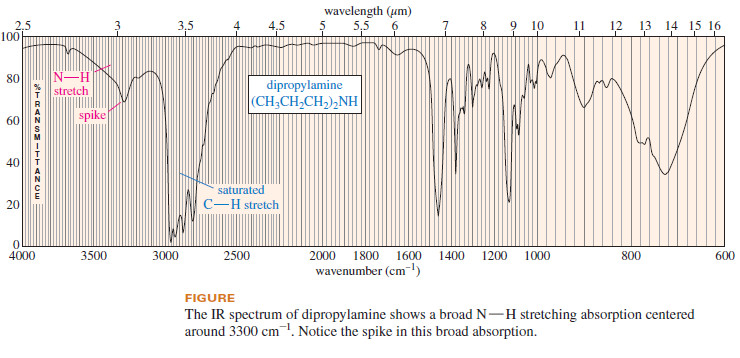
– Amine N-H bonds also have stretching frequencies in the 3300 cm-1 region, or even slightly higher.
– Like alcohols, amines participate in hydrogen bonding that can broaden the N-H absorptions.
– With amines, however, the absorption is somewhat weaker, and there may be one or more sharp spikes superimposed on the broad N-H stretching absorption: often one N-H spike for the single N-H bond of a secondary amine (R2NH) and two N-H spikes for the symmetric and antisymmetric stretch of the two N-H bonds in a primary amine (RNH2)
– These sharp spikes, combined with the presence of nitrogen in the molecular formula, help to distinguish amines from alcohols.
– Tertiary amines (R3N) have no N-H bonds, and they do not give rise to N-H stretching absorptions in the IR spectrum.
– The following shows the spectrum of dipropylamine, a secondary amine.