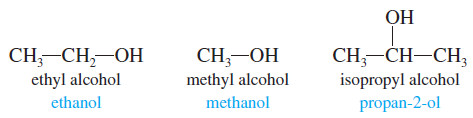Structure and Classification of Alcohols
– In this subject we will talk about Structure and Classification of Alcohols.
What are Alcohols?
– Alcohols are organic compounds containing hydroxyl (-OH) groups.
– They are some of the most common and useful compounds in nature, in industry, and around the house.
– The word alcohol is one of the oldest chemical terms, derived from the early Arabic al-kuhl.
– Originally it meant (the powder) and later (the essence). Ethyl alcohol, distilled from wine, was considered to be (the essence) of wine.
– Ethyl alcohol (grain alcohol) is found in alcoholic beverages, cosmetics, and drug preparations. Methyl alcohol (wood alcohol) is used as a fuel and solvent.
– Isopropyl alcohol (rubbing alcohol) is used as a skin cleanser for injections and minor cuts.
– Alcohols are synthesized by a wide variety of methods, and the hydroxyl group may be converted to most other functional groups.
– For these reasons, alcohols are versatile synthetic intermediates.
Structure and Classification of Alcohols
Structure of alcohols
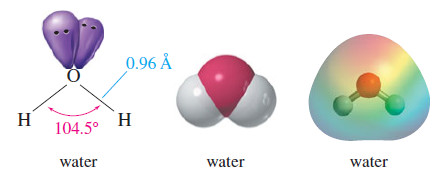
– The structure of an alcohol resembles the structure of water, with an alkyl group replacing one of the hydrogen atoms of water.
– The following Figure compares the structures of water and methanol.
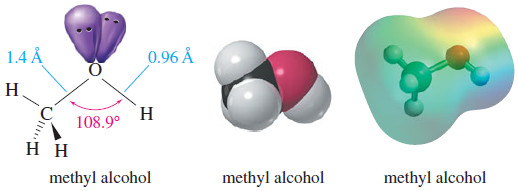
– Both have Sp3 – hybridized oxygen atoms, but the C-O-H bond angle in methanol (108.9°) is considerably larger than the H-O-H bond angle in water (104.5°) because the methyl group is much larger than a hydrogen atom.
– The bulky methyl group counteracts the bond angle compression caused by oxygen’s nonbonding pairs of electrons.
– The O-H bond lengths are about the same in water and methanol (0.96 Å), but the C-O bond is considerably longer (1.4 Å), reflecting the larger covalent radius of carbon compared to hydrogen.
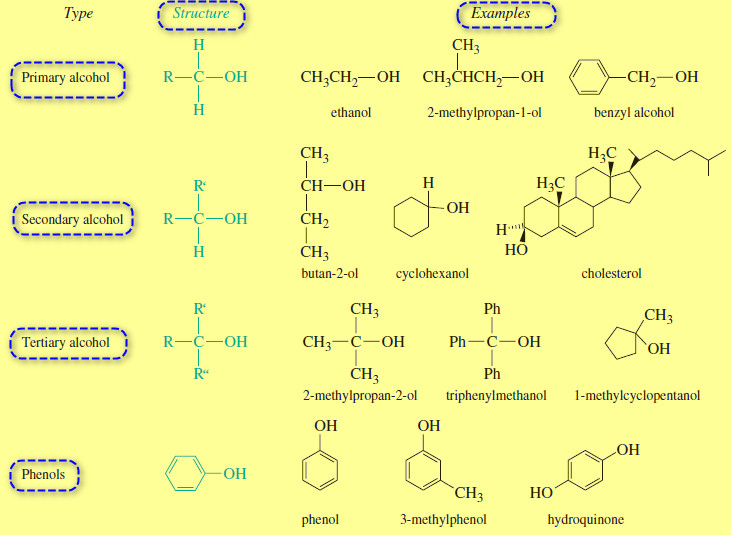
Classification of alcohols
– One way of organizing the alcohol family is to classify each alcohol according to the type of carbinol carbon atom: the one bonded to the -OH group.
– If this carbon atom is primary (bonded to one other carbon atom), the compound is a primary alcohol.
– A secondary alcohol has the -OH group attached to a secondary carbon atom, and a tertiary alcohol has it bonded to a tertiary carbon.
– When we studied alkyl halides, we saw that primary, secondary, and tertiary halides react differently. The same is true for alcohols.
– We need to learn how these classes of alcohols are similar and under what conditions they react differently.
– The following figure shows examples of primary, secondary, and tertiary alcohols.
– Compounds with a hydroxyl group bonded directly to an aromatic (benzene) ring are called phenols.
– Phenols have many properties similar to those of alcohols, while other properties derive from their aromatic character.