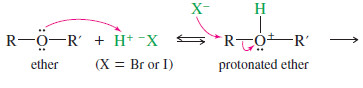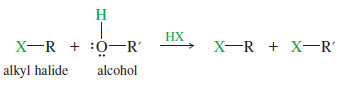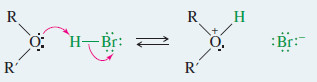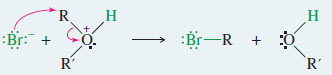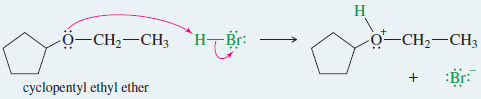Cleavage of Ethers by HBr and HI
– In this topic, we will discuss the Cleavage of Ethers by HBr and HI
Cleavage of Ethers by HBr and HI
– Unlike alcohols, ethers are not commonly used as synthetic intermediates because they do not undergo many reactions. This unreactivity makes ethers attractive as solvents. Even so, ethers do undergo a limited number of characteristic reactions.
– Ethers are cleaved by heating with HBr or HI to give alkyl bromides or alkyl iodides.
– Ethers are unreactive toward most bases, but they can react under acidic conditions.
– A protonated ether can undergo substitution or elimination with an alcohol serving as a neutral leaving group.
– Ethers react with concentrated HBr and HI because these reagents are sufficiently acidic to protonate the ether, while bromide and iodide are good nucleophiles for the substitution.
– Under these conditions, the alcohol leaving group usually reacts further with HX to give another alkyl halide.
– In effect, this reaction converts a dialkyl ether into two alkyl halides.
– The conditions are very strong, however, and the molecule must not contain any acid-sensitive functional groups.
– Iodide and bromide ions are good nucleophiles but weak bases, so they are more likely to substitute by SN2 the mechanism than to promote elimination by the E2 mechanism.
– The following Mechanism shows how bromide ion cleaves the protonated ether by displacing an alcohol.
– In the following example, cyclopentyl ethyl ether reacts with HBr to produce cyclopentanol by this displacement.
– Cyclopentanol reacts further with HBr, though, so the final products are ethyl bromide and bromocyclopentane.
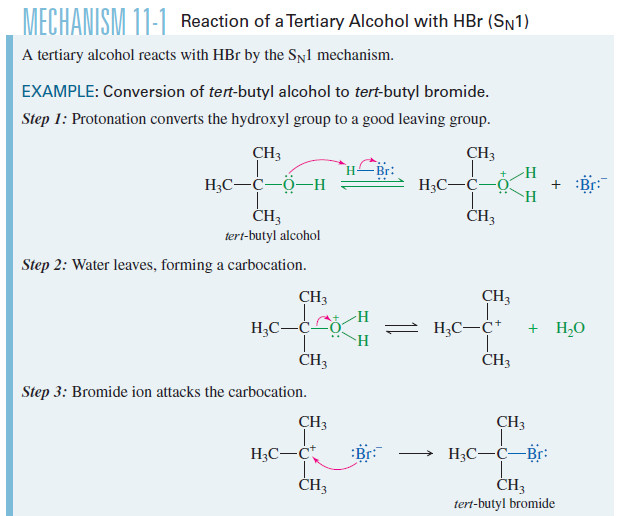
Mechanism: Cleavage of Ethers by HBr or HI
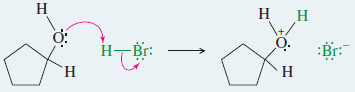
– Ethers are cleaved by a nucleophilic substitution of or on the protonated ether.
Step 1: Protonation of the ether to form a good leaving group
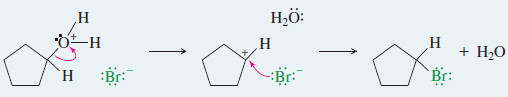
Step2: SN2 cleavage of the protonated ether.
Step 3: Conversion of the alcohol fragment to the alkyl halide. (Does not occur with phenols.)
– This conversion can occur by either of the two mechanisms, depending on the structure of the alcohol and the reaction conditions.
– The protonated alcohol undergoes either SN1 or SN2 substitution by bromide ion.
Example: Cleavage of cyclopentyl ethyl ether by HBr.
Step 1: Protonation of the ether to form a good leaving group.
Step2: Cleavage of the protonated ether.
Step 3: Conversion of the alcohol fragment to the alkyl halide. First, the alcohol is protonated to form a good leaving group.
The protonated alcohol undergoes SN1 or SN2 substitution by bromide ion.
– Hydroiodic acid (HI) reacts with ethers the same way HBr does.
– Aqueous iodide is a stronger nucleophile than aqueous bromide, and iodide reacts at a faster rate.
– We can rank the hydrohalic acids in order of their reactivity toward the cleavage of ethers:
HI >HBr >> HCl
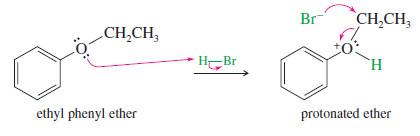
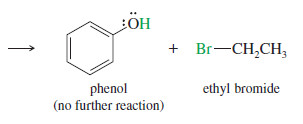
– Phenyl Ethers Phenyl ethers (one of the groups bonded to oxygen is a benzene ring) react with HBr or HI to give alkyl halides and phenols.
– Phenols do not react further to give halides because the sp2-hybridized carbon atom of the phenol cannot undergo the SN2 (or SN1) reaction needed for conversion to the halide.
References:
- Organic chemistry / L.G. Wade, Jr / 8th ed, 2013 / Pearson Education, Inc. USA.
- Fundamental of Organic Chemistry / John McMurry, Cornell University/ 8th ed, 2016 / Cengage Learningm, Inc. USA.
- Organic Chemistry / T.W. Graham Solomons, Craig B. Fryhle , Scott A. Snyder / 11 ed, 2014/ John Wiley & Sons, Inc. USA.