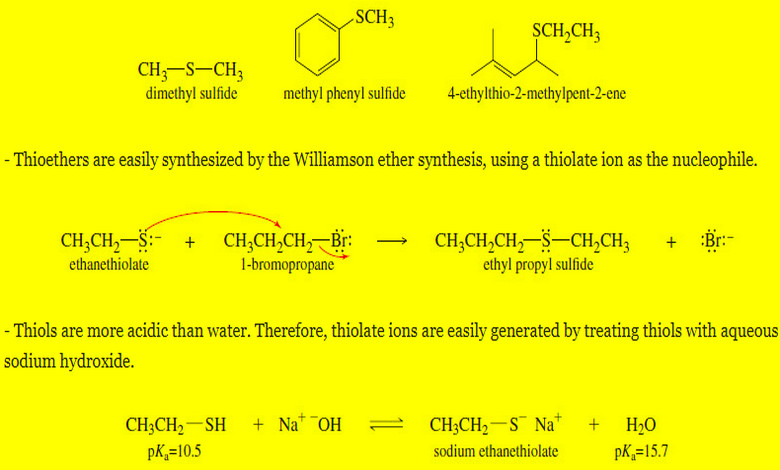
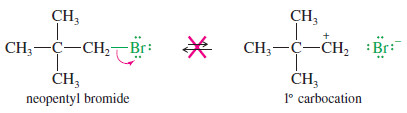Rearrangements in the SN1 Reactions
Rearrangements in the SN1 Reactions
– Carbocations frequently undergo structural changes, called rearrangements, to form more stable ions.
– A rearrangement may occur after a carbocation has formed or it may occur as the leaving group is leaving.
– Rearrangements are not seen in SN2 reactions, where no carbocation is formed and the one-step mechanism allows no opportunity for rearrangement.
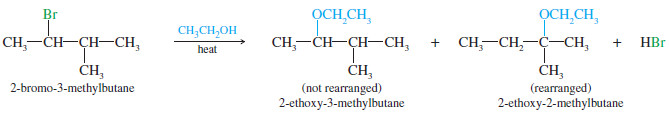
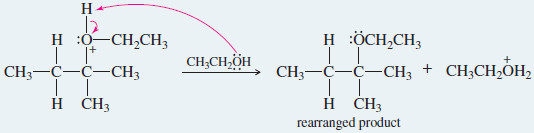
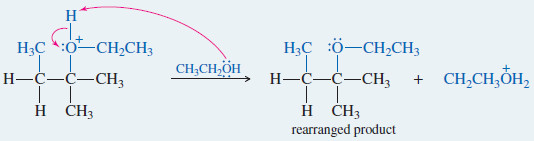
– An example of a reaction with rearrangement is the SN1 reaction of 2-bromo-3-methylbutane in boiling ethanol.
– The product is a mixture of 2-ethoxy 3-methylbutane (not rearranged) and 2-ethoxy-2-methylbutane (rearranged).
– The rearranged product, 2-ethoxy-2-methylbutane, results from a hydride shift, the movement of a hydrogen atom with its bonding pair of electrons.
– A hydride shift is represented by the symbol (~H)
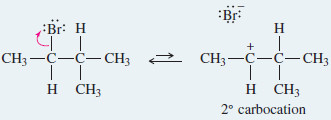
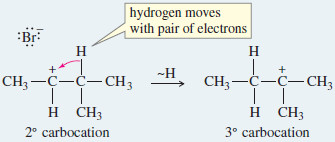
– In this case, the hydride shift converts the initially formed secondary carbocation to a more stable tertiary carbocation.
– Attack by the solvent gives the rearranged product.
MECHANISM(1): Hydride Shift in an SN1 Reaction
– Carbocations often rearrange to form more stable carbocations.
– This may occur when a hydrogen atom moves with its bonding pair of electrons.
– Formally, this is the movement of a hydride ion although no actual free hydride ion is involved.
Step 1: Unimolecular ionization gives a carbocation
Step 2: A hydride shift forms a more stable carbocation.
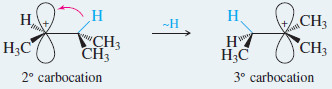
– This rearrangement involves movement of a hydrogen atom with its bonding pair of electrons over to the empty (p) orbital of the carbocation.
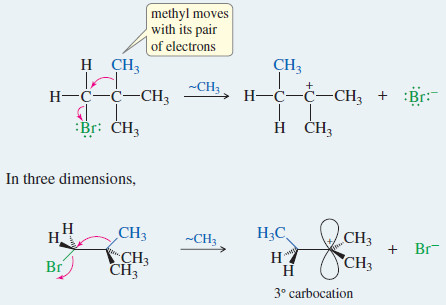
– In three dimensions, the rearrangement looks like this:
Step 3: Solvent (a weak nucleophile) attacks the rearranged carbocation
Step 4: Deprotonation gives the rearranged product.
– When neopentyl bromide is boiled in ethanol, it gives only a rearranged substitution product.
– This product results from a methyl shift (represented by the symbol (~CH3), the migration of a methyl group together with its pair of electrons.
– Without rearrangement, ionization of neopentyl bromide would give a very unstable primary carbocation.
The methyl shift occurs while bromide ion is leaving, so that only the more stable tertiary carbocation is formed.
MECHANISM(2): Methyl Shift in an SN1 Reaction
An alkyl group can rearrange to make a carbocation more stable.
Step 1: Ionization occurs with a methyl shift.
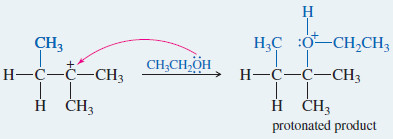
Step. 2: Attack by ethanol gives a protonated version of the rearranged product
Step 3: Deprotonation gives the rearranged product.
– Because rearrangement is required for ionization, only rearranged products are observed.
– In general, we should expect rearrangements in reactions involving carbocations whenever a hydride shift or an alkyl shift can form a more stable carbocation.
– Most rearrangements convert 2° (or incipient 1°) carbocations to 3° or resonancestabilized carbocations