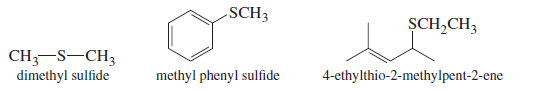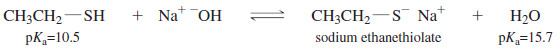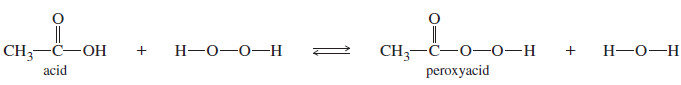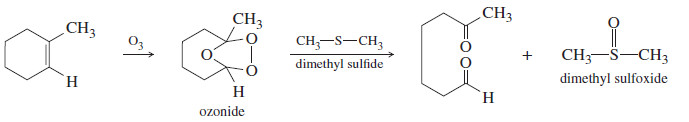Thioethers (sulfides) and Silyl Ethers
Thioethers (sulfides) and Silyl Ethers
– Thioethers, also called sulfides, are ethers with a sulfur atom replacing the oxygen atom of an ether, just like the sulfur in a thiol replaces the oxygen atom of an alcohol.
– The chemistry of thioethers is much like the chemistry of ethers, except that thioethers can undergo oxidation and alkylation of the sulfur atom.
– Silyl ethers are ethers with a substituted silicon atom replacing one of the alkyl groups of an ether.
– Silyl ethers share some of the properties of ethers (resistant to some acids, bases, and oxidizing agents), but they are more easily formed and more easily hydrolyzed.
– These properties make them useful as protecting groups, and silyl ethers are frequently used to protect alcohols.
Thioethers (Sulfides)
– Like thiols, thioethers have strong characteristic odors: The odor of dimethyl sulfide is reminiscent of oysters that have been kept in the refrigerator for too long.
– Sulfides are named like ethers, with “sulfide” replacing “ether” in the common names.
– In the IUPAC (alkoxy alkane) names, “alkylthio” replaces “alkoxy.”
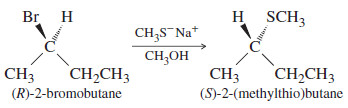
– Thioethers are easily synthesized by the Williamson ether synthesis, using a thiolate ion as the nucleophile.
– Thiols are more acidic than water. Therefore, thiolate ions are easily generated by treating thiols with aqueous sodium hydroxide.
– Because sulfur is larger and more polarizable than oxygen, thiolate ions are even better nucleophiles than alkoxide ions.
– Thiolates are such effective nucleophiles that secondary alkyl halides often react to give good yields of SN2 products.
– Sulfides are much more reactive than ethers.
– In a sulfide, sulfur valence is not necessarily filled: Sulfur can form additional bonds with other atoms.
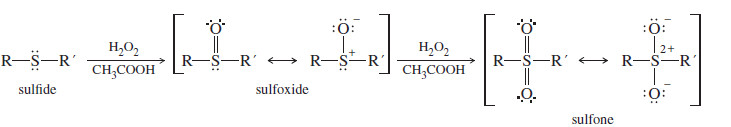
– Sulfur forms particularly strong bonds with oxygen, and sulfides are easily oxidized to sulfoxides and sulfones.
– Sulfoxides and sulfones are drawn using either hypervalent double-bonded structures or formally charged single-bonded structures as shown here.
– The hydrogen peroxide/acetic acid combination is a good oxidant for sulfides.
– One equivalent of peroxide gives the sulfoxide, and a second equivalent further oxidizes the sulfoxide to the sulfone.
– This reagent combination probably reacts via the peroxyacid, which is formed in equilibrium with hydrogen peroxide.
– Because they are easily oxidized, sulfides are often used as mild reducing agents.
– For example, we have used dimethyl sulfide to reduce the potentially explosive ozonides that result from ozonolysis of alkenes.
– Sulfur compounds are more nucleophilic than the corresponding oxygen compounds, because sulfur is larger and more polarizable and its electrons are less tightly held in orbitals that are farther from the nucleus.
– Although ethers are weak nucleophiles, sulfides are relatively strong nucleophiles.
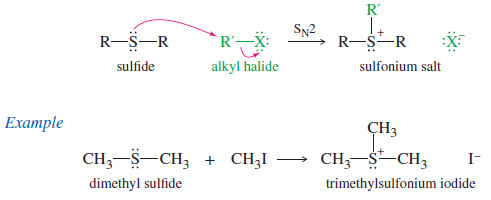
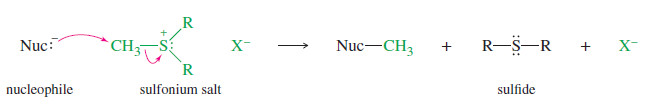
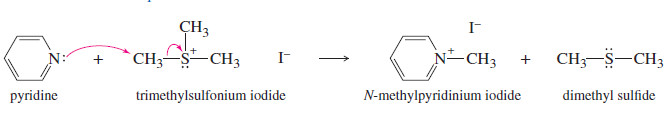
– Sulfides attack unhindered alkyl halides to give sulfonium salts.
– Sulfonium salts are strong alkylating agents because the leaving group is an uncharged
sulfide.
– Sulfur’s polarizability enhances partial bonding in the transition state, lowering its energy.
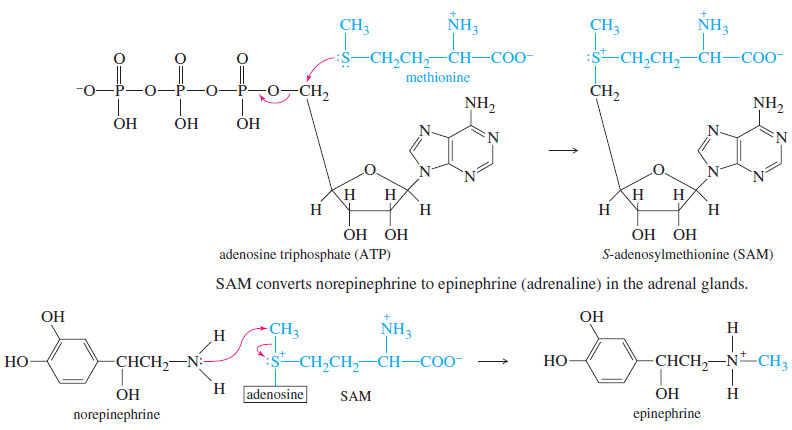
– Sulfonium salts are common alkylating agents in biological systems.
– For example, ATP activation of methionine forms the sulfonium salt S-adenosylmethionine (SAM), a biological methylating agent.
Silyl Ethers as Alcohol-Protecting Groups
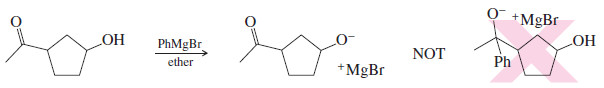
– If we have a compound with two or more functional groups, and we would like to modify just one of those functional groups, we often must protect any other functional groups to prevent them from reacting as well.
– For example, if we wanted to add a Grignard reagent to the carbonyl group of a keto alcohol, the alcohol group would protonate the Grignard reagent and the reaction would fail.
– Alcohol functional groups are common and useful, but they react with acids, bases, and oxidizing agents.
– Alcohols must be protected if they are to survive a reaction at another functional group on the molecule.
– A good protecting group must be easy to add to the group it protects, and then it must be resistant to the reagents used to modify other parts of the molecule.
– Finally, a good protecting group must be easy to remove to regenerate the original functional group.
– To accomplish the Grignard reaction shown above, we would need to convert the hydroxyl group to something that is resistant to Grignard reagents.
– For example, we might consider using an ether to protect a hydroxyl group in a Grignard reaction
– An ether protecting group can be difficult to remove (deprotect). It often requires strong acid, which can react with the free hydroxyl group or other parts of the molecule.
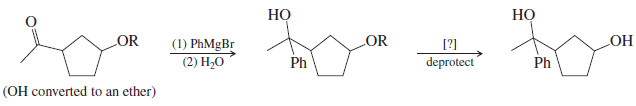
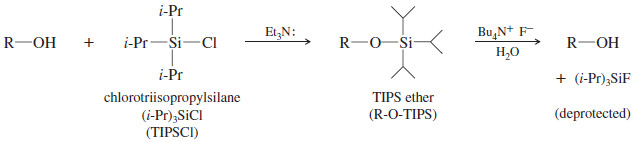
– Ethers based on silicon (silyl ethers) are much easier to remove than carbon-based ethers.
– In aqueous or organic solvents, fluoride ion removes silyl ethers under gentle conditions because the silicon–fluorine bond is exceptionally strong.
Synthetic organic chemists have developed many different silyl protecting groups that vary widely in their reactivity and are carefully chosen for a specific use.
– We will use the triisopropylsilyl (Tri-Iso-Propyl-Silyl or TIPS) protecting group, of structure R-O-Si(i-Pr)3 as our example.
– The three bulky isopropyl groups stabilize this silyl ether by hindering attack by nucleophiles.
– Silyl ethers are commonly formed by the reaction of alcohols with chlorosilanes in the presence of tertiary amines.
– We can form a TIPS ether by a reaction of chlorotriisopropylsilane (TIPSCl) with a tertiary amine such as triethylamine (Et3N:).
– TIPS ethers are stable to most acids and bases and oxidizing and reducing agents.
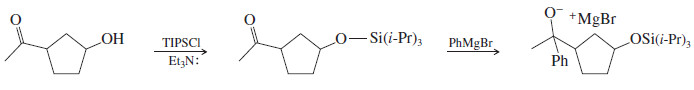
– Our keto-alcohol shown above would react with TIPS chloride (TIPSCl) and triethylamine (Et3N:) to give a protected alcohol.
– In our example, we can add a Grignard reagent to the carbonyl group in the presence of the protected alcohol.
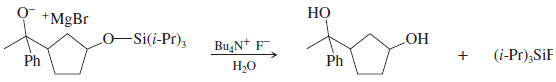
– After the Grignard reaction is completed, protonation of the magnesium alkoxide salt and deprotection of the silyl ether gives the desired product.
References:
- Organic chemistry / L.G. Wade, Jr / 8th ed, 2013 / Pearson Education, Inc. USA.
- Fundamental of Organic Chemistry / John McMurry, Cornell University/ 8th ed, 2016 / Cengage Learningm, Inc. USA.
- Organic Chemistry / T.W. Graham Solomons, Craig B. Fryhle , Scott A. Snyder / 11 ed, 2014/ John Wiley & Sons, Inc. USA.