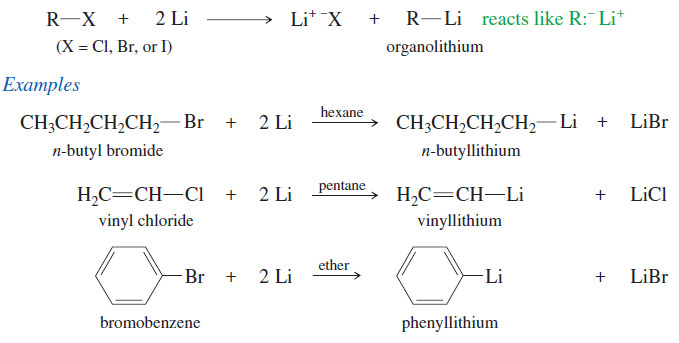
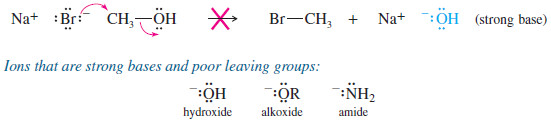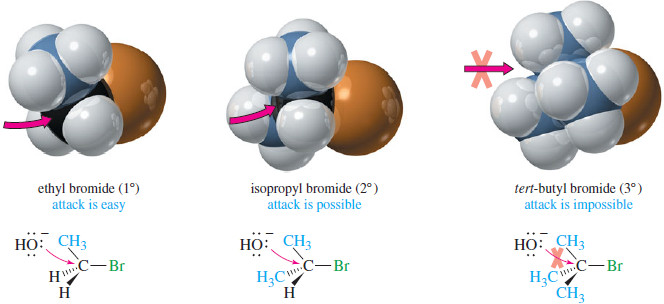Reactivity of the Substrate in SN2 Reactions
Reactivity of the Substrate in SN2 Reactions
– We will often refer to the alkyl halide as the substrate: literally, the compound that is being attacked by the reagent.
– Just as the nucleophile is important in the SN2 reaction, the structure of the alkyl halide is equally important.
– Besides alkyl halides, a variety of other types of compounds serve as substrates in SN2 reactions.
– To be a good substrate for SN2 attack by a nucleophile, a molecule must have an electrophilic carbon atom with a good leaving group, and that carbon atom must not be too sterically hindered for a nucleophile to attack.
Leaving-Group Effects on the Substrate
– A leaving group serves two purposes in the reaction:
1. It polarizes the C-X bond, making the carbon atom electrophilic.
2. It leaves with the pair of electrons that once bonded it to the electrophilic carbon atom.
– To fill these roles, a good leaving group should be
1. electron withdrawing, to polarize the carbon atom,
2. stable (not a strong base) once it has left, and
3. polarizable, to stabilize the transition state.
(1) The leaving group must be electron withdrawing
– The leaving group must be electron withdrawing to create a partial positive charge on the carbon atom, making the carbon electrophilic.
– An electron-withdrawing leaving group also stabilizes the negatively charged transition state.
– Halogen atoms are strongly electronegative, so alkyl halides are common substrates for SN2 reactions.
– Oxygen, nitrogen, and sulfur also form strongly polarized bonds with carbon; given the right substituents, they can form the basis for excellent leaving groups.
Strongly polarized
(2) The leaving group must be stable
– The leaving group must be stable once it has left with the pair of electrons that bonded it to carbon.
– A stable leaving group is needed for favorable energetics.
– The leaving group is leaving in the transition state; a reactive leaving group would raise the energy of the transition state, slowing the reaction.
– Also, the energy of the leaving group is reflected in the energy of the products.
– A reactive leaving group would raise the energy of the products, driving the equilibrium toward the reactants.
– Good leaving groups should be weak bases; therefore, they are the conjugate bases of strong acids.
– The hydrohalic acids HCl, HBr, and HI are strong, and their conjugates bases (Cl–, Br– and I–) are all weak bases.
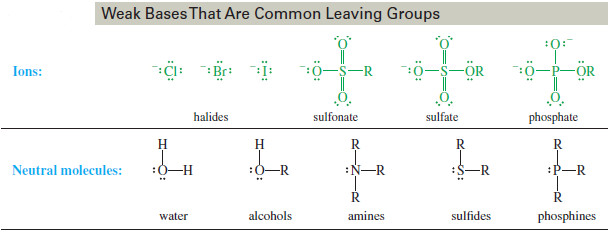
– Other weak bases, such as sulfate ions, sulfonate ions, and phosphate ions, can also serve as good leaving groups.
– The following table lists examples of good leaving groups.
– Hydroxide ion, alkoxide ions, and other strong bases are poor leaving groups for SN2 reactions.
– For example, the O-H group of an alcohol is a poor leaving group because it would have to leave as hydroxide ion.
– The previous Table also lists some neutral molecules that can be good leaving groups.
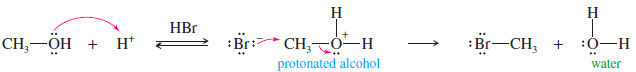
– A neutral molecule often serves as the leaving group from a positively charged species.
– For example, if an alcohol is placed in an acidic solution, the hydroxyl group is protonated.Water then serves as the leaving group.
– Note that the need to protonate the alcohol (requiring acid) limits the choice of nucleophiles to those few that are weak bases, such as bromide and iodide.
– A strongly basic nucleophile would become protonated in acid
(3) A good leaving group should be polarizable
– Finally, a good leaving group should be polarizable, to maintain partial bonding with the carbon atom in the transition state.
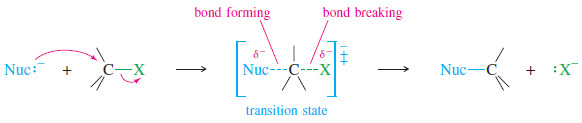
– This bonding helps stabilize the transition state and reduce the activation energy.
– The departure of a leaving group is much like the attack of a nucleophile, except that the bond is breaking rather than forming.
– Polarizable nucleophiles and polarizable leaving groups both stabilize the transition state by engaging in more bonding at a longer distance.
– Iodide ion, one of the most polarizable ions, is both a good nucleophile and a good leaving group.
– In contrast, fluoride ion is a small, “hard” ion. Fluoride is both a poor nucleophile (in protic solvents) and a poor leaving group in SN2 reactions.
Steric Effects on the Substrate
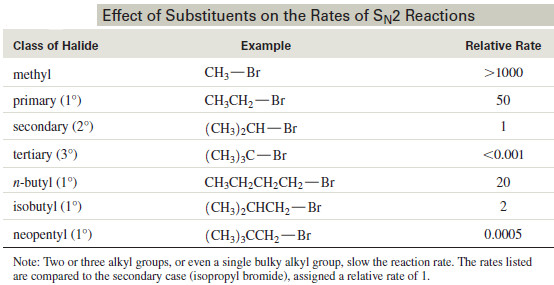
– Different alkyl halides undergo reactions at vastly different rates.
– The structure of the substrate is the most important factor in its reactivity toward displacement.
– The reaction goes rapidly with methyl halides and with most primary substrates.
– It is more sluggish with secondary halides.
– Tertiary halides fail to react at all by the mechanism.
– The following Table shows the effect of alkyl substitution on the rate of displacements.
– For simple alkyl halides, the relative rates for displacement are Relative rates for SN2:
CH3X > 1° > 2° >> 3°
– The physical explanation for this order of reactivity is suggested by the information in the Table .
– All the slow-reacting compounds have one property in common: The back side of the electrophilic carbon atom is crowded by the presence of bulky groups.
– Tertiary halides are more hindered than secondary halides, which are more hindered than primary halides.
– Even a bulky primary halide (like neopentyl bromide) undergoes SN2 reaction at a rate similar to that of a tertiary halide.
– The relative rates show that it is the bulk of the alkyl groups, rather than an electronic effect, that hinders the reactivity of bulky alkyl halides in the SN2 displacement.
– This effect on the rate is another example of steric hindrance.
Explanation
– When the nucleophile approaches the back side of the electrophilic carbon atom, it must come within bonding distance of the back lobe of the C- X sp3 orbital.
– If there are two alkyl groups bonded to the carbon atom, this process is difficult. Three alkyl groups make it impossible.
– Just one alkyl group can produce a large amount of steric hindrance if it is unusually bulky, like the tert-butyl group of neopentyl bromide.
– The following Figure shows the SN2 reaction of hydroxide ion with ethyl bromide (1°), isopropyl bromide (2°), and tert-butyl bromide (3°).
– The nucleophile can easily approach the electrophilic carbon atom of ethyl bromide.
– In isopropyl bromide, the approach is hindered, but still possible.
– In contrast, SN2 approach to the tertiary carbon of tert-butyl bromide is impossible because of the steric hindrance of the three methyl groups.
– Make models of ethyl bromide, isopropyl bromide, and tert-butyl romide, and compare the ease of bringing in an atom for a back-side attack.