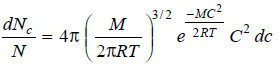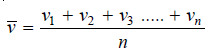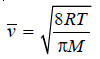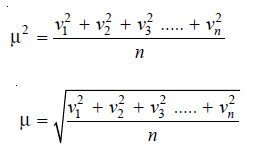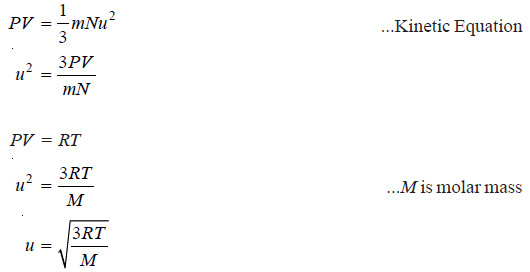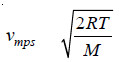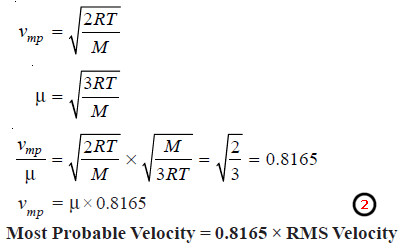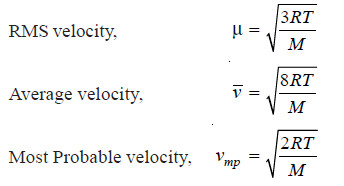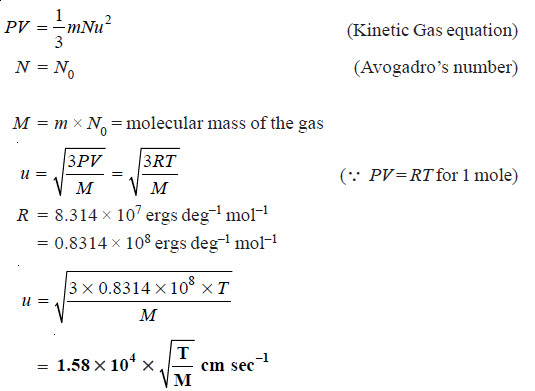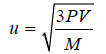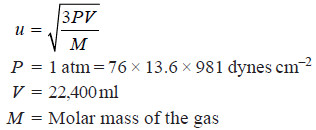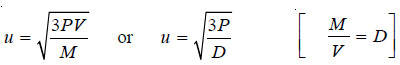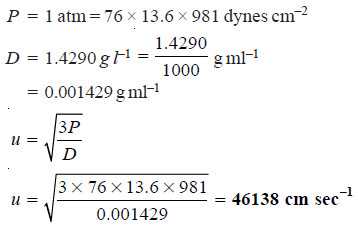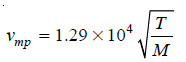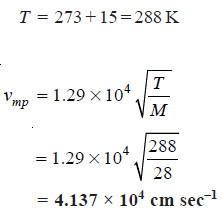Distribution of Molecular velocities
– In this topic, we will discuss Distribution of Molecular velocities.
Distribution of Molecular velocities
– While deriving Kinetic Gas Equation, it was assumed that all molecules in a gas have the same velocity. But it is not so.
– When any two molecules collide, one molecule transfers kinetic energy (1/2 mv2) to the other molecule.
– The velocity of the molecule which gains energy increases and that of the other decreases.
– Millions of such molecular collisions are taking place per second. Therefore, the velocities of molecules are changing constantly.
– Since the number of molecules is very large, a fraction of molecules will have the same particular velocity.
– In this way there is a broad distribution of velocities over different fractions of molecules.
– In 1860 James Clark Maxwell calculated the distribution of velocities from the laws of probability.
– He derived the following equation for the distribution of molecular velocities.
where:
dNc = number of molecules having velocities between C and (C + dc)
N = total number of molecules
M = molecular mass
T = temperature on absolute scale (K)
– The relation stated above is called Maxwell’s law of distribution of velocities.
– The ratio dn/n gives the fraction of the total number of molecules having velocities between C and (C + dc).
– Maxwell plotted such fractions against velocity possessed by the molecules.
– The curves so obtained illustrate the salient features of Maxwell distribution of velocities.
Distribution of velocities in Nitrogen gas
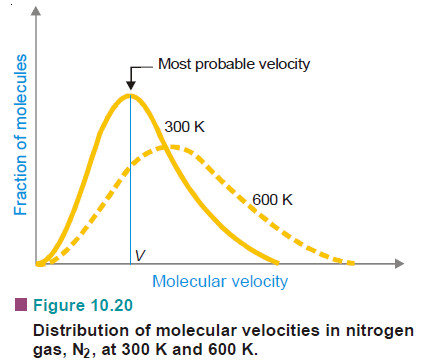
– The following Fig Shows the distribution of velocities in nitrogen gas, N2, at 300 K and 600 K. It will be noticed that :
(1) A very small fraction of molecules has either very low (close to zero) or very high velocities.
(2) Most intermediate fractions of molecules have velocities close to an average velocity represented by the peak of the curve. This velocity is called the most probable velocity. It may be defined as the velocity possessed by the largest fraction of molecules corresponding to the highest point on the Maxvellian curve.
(3) At higher temperature, the whole curve shifts to the right (dotted curve at 600 K). This shows that at higher temperature more molecules have higher velocities and fewer molecules have lower velocities.
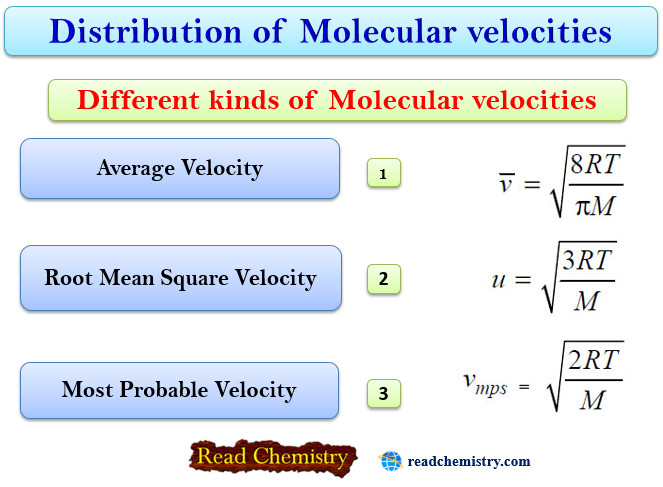
Different kinds of Molecular velocities
– In our study of kinetic theory we come across three different kinds of molecular velocities :
(1) the Average velocity (V)
(2) the Root Mean Square velocity (μ)
(3) the Most Probable velocity (vmn)
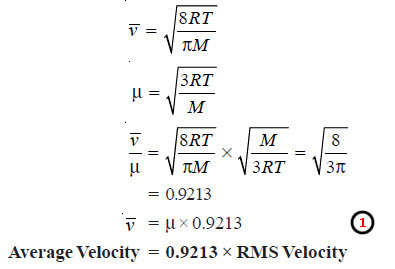
Average Velocity
– Let there be n molecules of a gas having individual velocities v1, v2, v3 ….. vn.
– The ordinary average velocity is the arithmetic mean of the various velocities of the molecules.
– From Maxwell equation it has been established that the average velocity vav is given by the expression:
– Substituting the values of R, T, π and M in this expression, the average value can be calculated.
Root Mean Square Velocity
– If v1, v2, v3 ….. vn are the velocities of n molecules in a gas, μ2, the mean of the squares of all the velocities is:
– μ is thus the Root Mean Square velocity or RMS velocity. It is denoted by u.
– The value of the RMS of velocity u, at a given temperature can be calculated from the Kinetic Gas Equation.
– By substituting the values of R, T and M, the value of u (RMS velocity) can be determined.
– RMS velocity is superior to the average velocity considered earlier.
– With the help of u, the total Kinetic energy of a gas sample can be calculated.
Most Probable Velocity
– As already stated the most probable velocity is possessed by the largest number of molecules in a gas.
– According to the calculations made by Maxwell, the most probably velocity, vmp, is given by the expression.
– Substituting the values of R, T and M in this expression, the most probably velocity can be calculated.
Relation between Average Velocity, RMS Velocity and Most Probable Velocity
– We know that the average velocity, , is given by the expression:
– The expression for the most probably velocity, vmp, is:
– RMS can be easily calculated by the application of Kinetic Gas equation.
– Knowing the value of RMS, we can find the average velocity and the most probable velocity from expressions (1) and
(2).
Calculation of Molecular velocities
– The velocities of gas molecules are exceptionally high. Thus velocity of hydrogen molecule is 1,838 metres sec–1.
– While it may appear impossible to measure so high velocities, these can be easily calculated from the Kinetic Gas equation.
– Several cases may arise according to the available data.
– While calculating different types of velocities, we can also make use of the following expressions stated already.
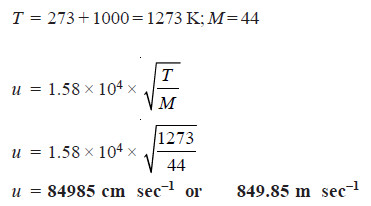
Case (1): Calculation of Molecular Velocities when temperature alone is given
– where T is Kelvin temperature and M the molar mass.
Solved problem: Calculate the root mean square velocity of CO2 molecule at 1000°C.
Solution:
Case (2): Calculation of Molecular Velocity when temperature and pressure both are given.
In such cases we make use of the following relation based on Kinetic Gas equation.
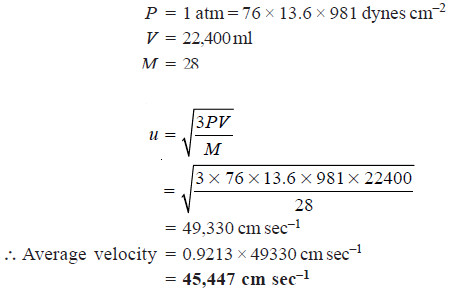
– We know that 1 mole of a gas at STP occupies a volume of 22400 ml (known as molar volume).
– But before applying this relation the molar volume is reduced to the given conditions of temperature and pressure.
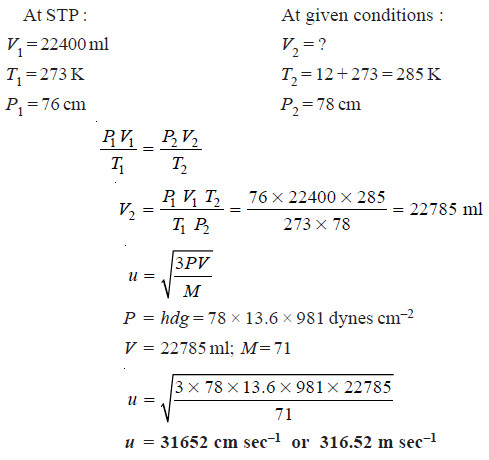
Solved problem: Calculate the RMS velocity of chlorine molecules at 12°C and 78 cm pressure
Solution:
Case (3): Calculation of Molecular Velocity at STP
– Here we use the relation:
Solved problem: Calculate the average velocity of nitrogen molecule at STP.
Solution:
Case (4) Calculation of Molecular Velocity when pressure and density are given
– In this case we have:
– where P is expressed in dynes cm–2 and D in gm ml–1.
– Find the RMS velocity of oxygen molecules.
Solved problem: Oxygen at 1 atmosphere pressure and 0°C has a density of 1.4290 grams per litre.
Solution:
Case (5): Calculation of most probable velocity
– In this case we have:
where T expressed in Kelvin and M to mass.
Solved problem: Calculate the most probable velocity of nitrogen molecules, N2, at 15°C.
Solution:
References
- Atkins’ Physical Chemistry / Peter Atkin, Julio de Paula, James Keeler / 12th edition, 2022 / Oxford University Press, UK.
- Physical Chemistry/ Robert G. Mortimer/ 3rd Edition / 2008/ Elsevier Inc, USA.
- Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolor edition/ S. Chand Publishing co / india.
- Physical chemistry for the chemical sciences / Raymond Chang, John W. Thoman, Jr./1st edition, 2014/ University Science Books, USA