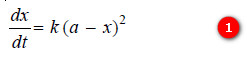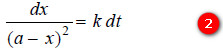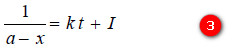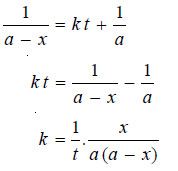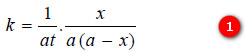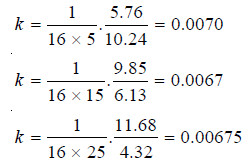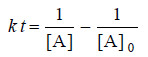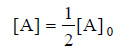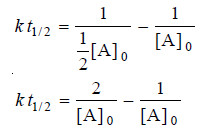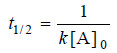Second order reaction
Second order reaction
– Let us take a second order reaction of the type
2A ⎯⎯→ products
– Suppose the initial concentration of A is a moles liter–1.
– If after time t, x moles of A have reacted, the concentration of A is (a – x).
– We know that for such a second order reaction, the rate of reaction is proportional to the square of the concentration of the reactant. Thus,
where k is the rate constant, Rearranging equation (1), we have
On integration, it gives
where I is an integration constant. I can be evaluated by putting x = 0 and t = 0. Thus,
Substituting for I in equation (3)
This is the integrated rate equation for a second order reaction.
Examples of Second order Reaction
– Hydrolysis of an Ester by NaOH. This is a typical second order reaction.
CH3COOC2H5 + NaOH ⎯⎯→ CH3COONa + C2H5OH
ethyl acetate ethyl alcohol
– The reaction is carried in a vessel at a constant temperature by taking. equimolar amounts of ethyl acetate and NaOH.
– Measured volumes of the reaction mixture (say, 25 ml) are withdrawn at various times and titrated against a standard acid.
– The volume of the acid used is a measure of the concentration of NaOH or ester.
– Thus the volume of the acid used when t = 0, gives the initial concentration (a) of the reactants.
– The volume of acid consumed at any other time t gives (a – x). The value of x can be calculated.
– The rate constant k can be determined by substituting values in the second-order integrated rate equation.
Solved problems
Solved problem (1): Hydrolysis of ethyl acetate by NaOH using equal concentration of the reactants was studied by titrating 25ml of the reaction mixture at different time intervals against standard acid. From the data given below, establish that this is a second-order reaction.
Solution:
The second order integrated rate equation is:
– The volume of acid used at any time is a measure of concentration of the unreacted substances at that time.
– Therefore,
a, initial concentration = 16.00
after 5 mts (a – x) = 10.24 and x = 5.76
and after 15 mts (a – x) = 6.13 and x = 9.85
after 25 mts (a – x) = 4.32 and x = 11.68
– Substituting values in the rate equation (1), we have:
– The values of k being fairly constant, this reaction is of the second order.
Units of Second order Rate constant
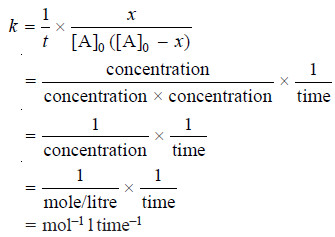
– The rate constant for a second order reaction is expressed as
– Thus the units for k for a second order reactions are: mol–1 l time–1
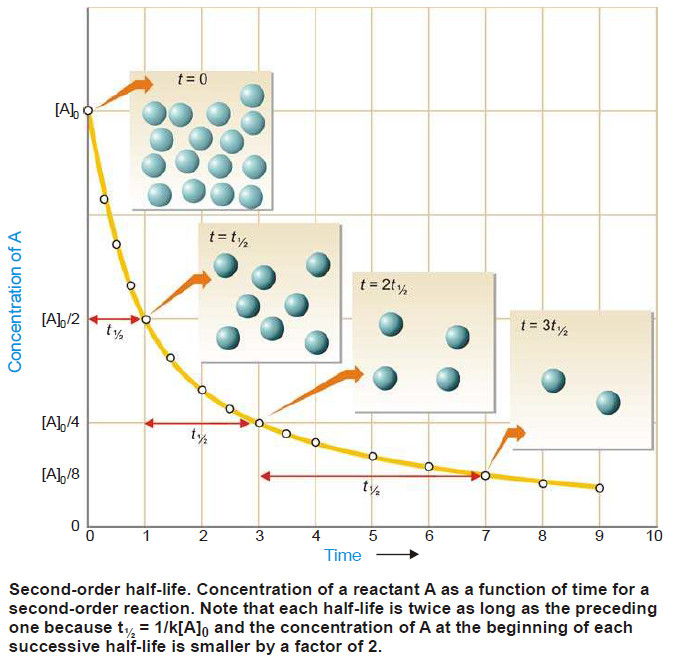
Calculation of Half-life of a second order Reaction
– Reaction rates can also be expressed in terms of half-life or half-life period.
– It is defined as the time required for the concentration of a reactant to decrease to half its initial value.
– In other words, half-life is the time required for one-half of the reaction to be completed.
– It is represented by the symbol t1/2 or t0.5.
– For the simple second order reaction 2A → Products, the integrated rate equation is:
– where [A]0 is the initial concentration and [A] is the concentration when time t has elapsed. When one-half life has elapsed.
– and we have
– Solving for t1/2 we find that
– As in case of a first order reaction, half-life for a second order reaction is inversely proportional to rate constant k.
– While half-life of a first order reaction is independent of initial concentration, half-life of a second order reaction depends on initial concentration.
– This fact can be used to distinguish between a first order and a second order reaction.