Heterocyclic Aromatic Compounds
Heterocyclic Aromatic Compounds
– Nitrogen, oxygen, and sulfur are the most common heteroatoms in heterocyclic aromatic compounds.
– The criteria for Hückel’s rule require a ring of atoms, all with unhybridized p orbitals overlapping in a continuous ring.
– In discussing aromaticity, we have considered only compounds composed of rings of sp2 hybrid carbon atoms.
– Heterocyclic compounds, with rings containing sp2-hybridized atoms of other elements, can also be aromatic.
– Nitrogen, oxygen, and sulfur are the most common heteroatoms in heterocyclic aromatic compounds.
Pyridine
– Pyridine is an aromatic nitrogen analogue of benzene.
– It has a six-membered heterocyclic ring with six pi electrons.
– Pyridine has a nitrogen atom in place of one of the six C-H units of benzene, and the nonbonding pair of electrons on nitrogen replaces benzene’s bond to a hydrogen atom.
– These nonbonding electrons are in an sp2 hybrid orbital in the plane of the ring (Figure). They are perpendicular to the pi system and do not overlap with it.
– Pyridine shows all the characteristics of aromatic compounds. It has a resonance energy of 113 kJ mol (27 kcal mol) and it usually undergoes substitution rather than addition.
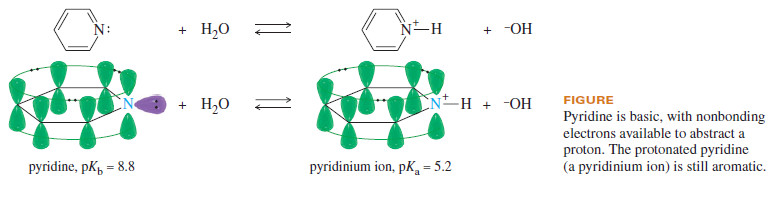
– Because it has an available pair of nonbonding electrons, pyridine is basic (Figure).
– In an acidic solution, pyridine protonates to give the pyridinium ion.
– The pyridinium ion is still aromatic because the additional proton has no effect on the electrons of the aromatic sextet: It simply bonds to pyridine’s nonbonding pair of electrons.
Pyrrole
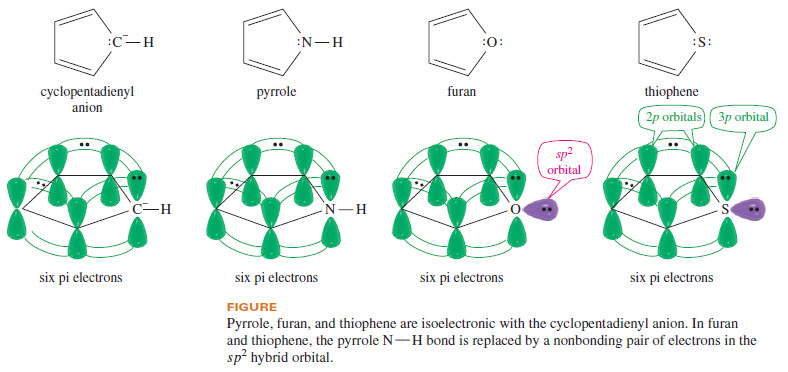
– Pyrrole is an aromatic five-membered heterocycle, with one nitrogen atom and two double bonds (Figure).
– Although it may seem that pyrrole has only four pi electrons, the nitrogen atom has a lone pair of electrons.
– The pyrrole nitrogen atom is sp2 hybridized, and its unhybridized p orbital overlaps with the p orbitals of the carbon atoms to form a continuous ring.
– The lone pair on nitrogen occupies the p orbital, and (unlike the lone pair of pyridine) these electrons take part in the pi bonding system.
– These two electrons, added to the four pi electrons of the two double bonds, complete an aromatic sextet.
– Pyrrole has a resonance energy of 92 kJ/mol (22 kcal/mol).
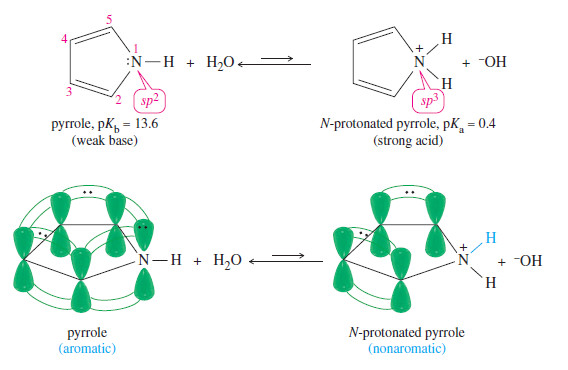
– Pyrrole (pkb = 13.6) is a much weaker base than pyridine(pkb = 8.8) .This difference is due to the structure of the protonated pyrrole (Figure).
– To form a bond to a proton requires the use of one of the electron pairs in the aromatic sextet.
– In the protonated pyrrole, the nitrogen atom is bonded to four different atoms (two carbon atoms and two hydrogen atoms), requiring sp3 hybridization and leaving no unhybridized p orbital.
– The protonated pyrrole is nonaromatic. In fact, a sufficiently strong acid actually protonates pyrrole at the 2-position, on one of the carbon atoms of the ring, rather than on nitrogen.
Pyrimidine and Imidazole
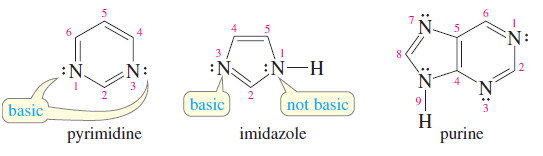
– Pyrimidine is a six-membered heterocycle with two nitrogen atoms situated in a 1,3- arrangement.
– Both nitrogen atoms are like the pyridine nitrogen.
– Each has its lone pair of electrons in the sp2 hybrid orbital in the plane of the aromatic ring.
– These lone pairs are not needed for the aromatic sextet, and they are basic, like the lone pair of pyridine.
– Imidazole is an aromatic five-membered heterocycle with two nitrogen atoms.
– The lone pair of one of the nitrogen atoms (the one not bonded to a hydrogen) is in an sp2 orbital that is not involved in the aromatic system; this lone pair is basic.
– The other nitrogen uses its third sp2 orbital to bond to hydrogen, and its lone pair is part of the aromatic sextet.
– Like the pyrrole nitrogen atom, this imidazole N-H nitrogen is not very basic.
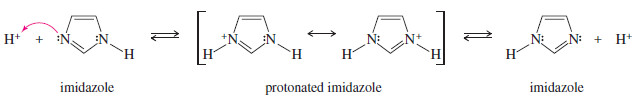
– Once imidazole is protonated, the two nitrogens become chemically equivalent.
– Either nitrogen can lose a proton and return to an imidazole molecule.
– Purine has an imidazole ring fused to a pyrimidine ring.
– Purine has three basic nitrogen atoms and one pyrrole-like nitrogen.
– Pyrimidine and purine derivatives serve in DNA and RNA to specify the genetic code.
– Imidazole derivatives enhance the catalytic activity of enzymes.
Furan and Thiophene
– Like pyrrole, furan is an aromatic five-membered heterocycle, but in furan the heteroatom is oxygen instead of nitrogen.
– The classical structure for furan (Figure) shows that the oxygen atom has two lone pairs of electrons.
– The oxygen atom is sp2 hybridized, and one of the lone pairs occupies an sp2 hybrid orbital.
– The other lone pair occupies the unhybridized p orbital, combining with the four electrons in the double bonds to give an aromatic sextet.
– Furan has a resonance energy of 67 kJ mol (16 kcal mol).
– Thiophene is similar to furan, with a sulfur atom in place of the furan oxygen.
– The bonding in thiophene is similar to that in furan, except that the sulfur atom uses an unhybridized 3p orbital to overlap with the 2p orbitals on the carbon atoms.
– The resonance energy of thiophene is 121 kJ/mol (29 kcal/mol).