-
Physical Chemistry
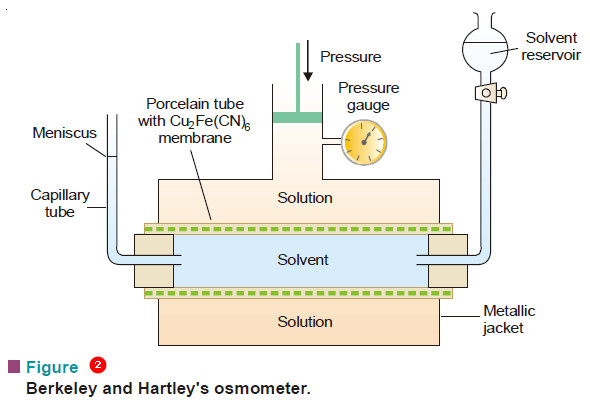
Determination of osmotic pressure
Determination of osmotic pressure – The osmotic pressure of a given solution can be determined experimentally by the methods detailed…
Read More » -
Physical Chemistry
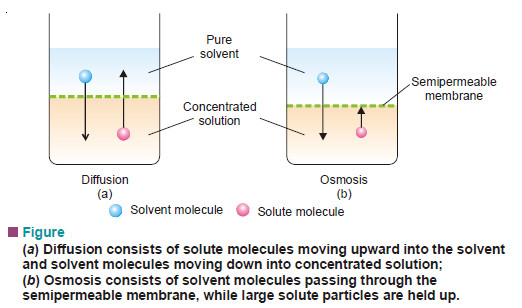
What is Osmosis and Osmotic Pressure?
Diffusion and Osmosis – Just as a gas can diffuse into vacant space or another gas, a solute can diffuse…
Read More » -
Physical Chemistry
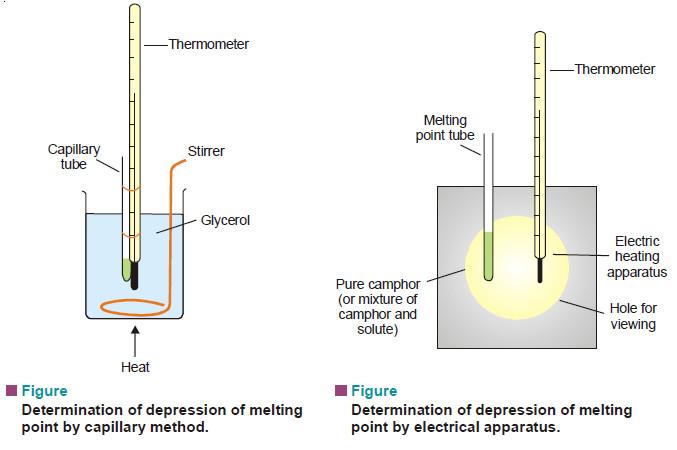
Measurement of freezing point Depression
The subject of Measurement of freezing point Depression will be discused FREEZING POINT DEPRESSION Relation between Depression of Freezing point…
Read More » -
Physical Chemistry
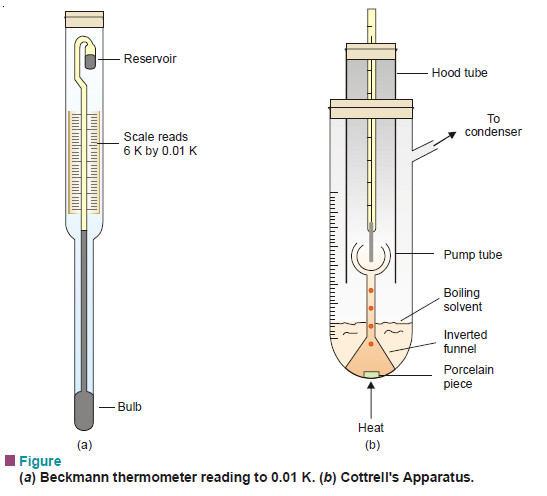
Measurement of boiling point elevation
MEASUREMENT OF BOILING POINT ELEVATION – There are several methods available for the measurement of the elevation of boiling point.…
Read More » -
Physical Chemistry
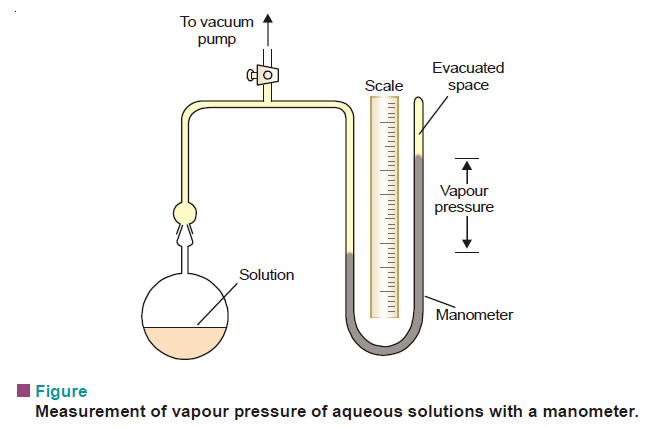
Measurement of lowering of vapour pressure
MEASUREMENT OF LOWERING OF VAPOUR PRESSURE (1) Barometric Method – Raoult measured the individual vapour pressure of a liquid and…
Read More » -
Physical Chemistry
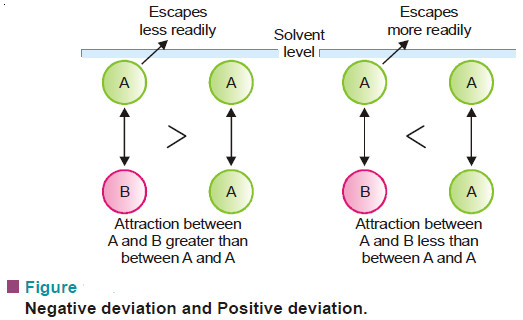
Lowering of vapour pressure- Raoult’s law
In this subject we will restrict our discussion to Raoult’s Law COLLIGATIVE PROPERTIES – Dilute solutions containing non-volatile solute exhibit…
Read More » -
Physical Chemistry
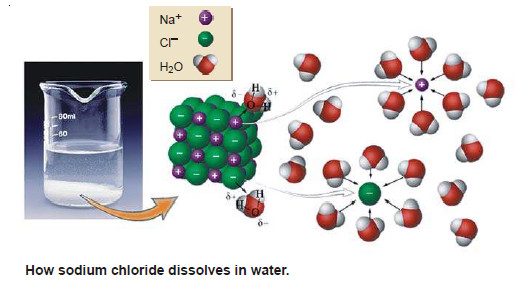
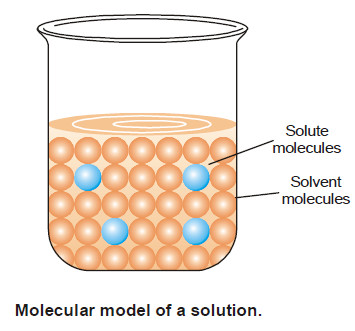
Solutions of solid substances in liquids
SOLUTIONS OF SOLIDS IN LIQUIDS – Solutions of a solid substance in a solvent are most commonly met with. –…
Read More » -
Uncategorized
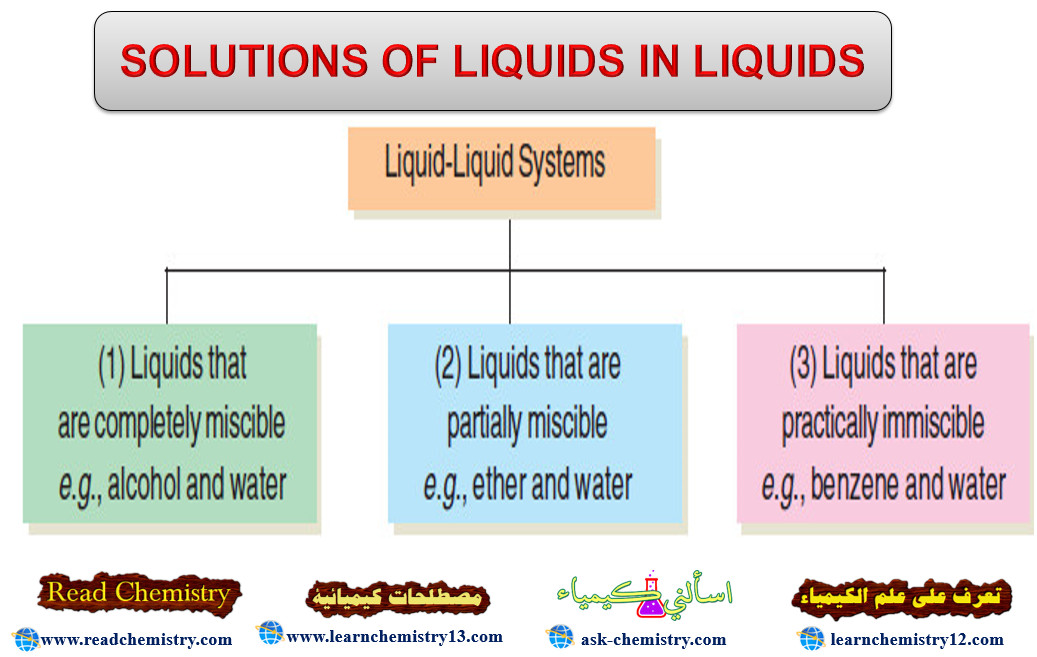
Solutions of liquids in liquids
SOLUTIONS OF LIQUIDS IN LIQUIDS – The solutions of liquids in liquids may be divided into three classes as follows:…
Read More » -
Physical Chemistry
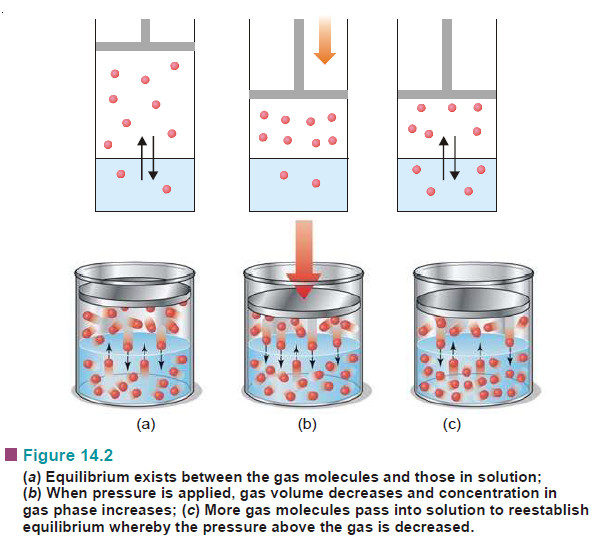
Henry’s Law – Solutions of gases in gases
The subject of Solutions of gases in gases – Henry’s Law will be discused Types of Solutions – The common…
Read More » -
Physical Chemistry
Ways of Expressing Concentration
Concentration of A Solution – The concentration of a solution is defined as : the amount of solute present in…
Read More » -
Physical Chemistry
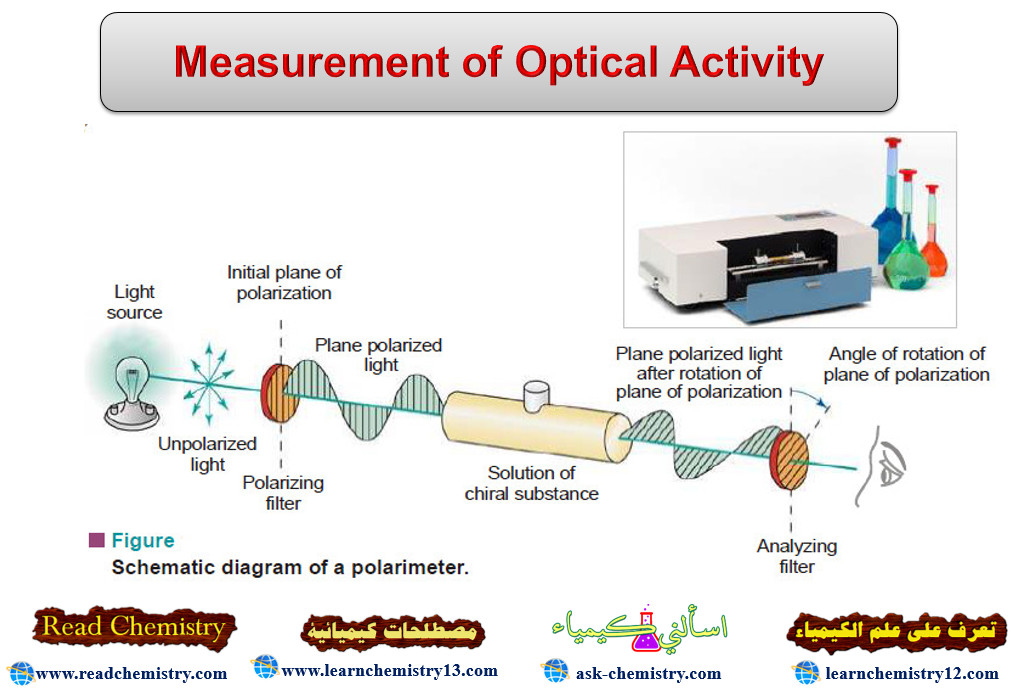
Measurement of Optical Activity
Optical Activity – Optical activity is one of imortant physcial properties of liqiuds – A beam of ordinary light consists…
Read More » -
Physical Chemistry
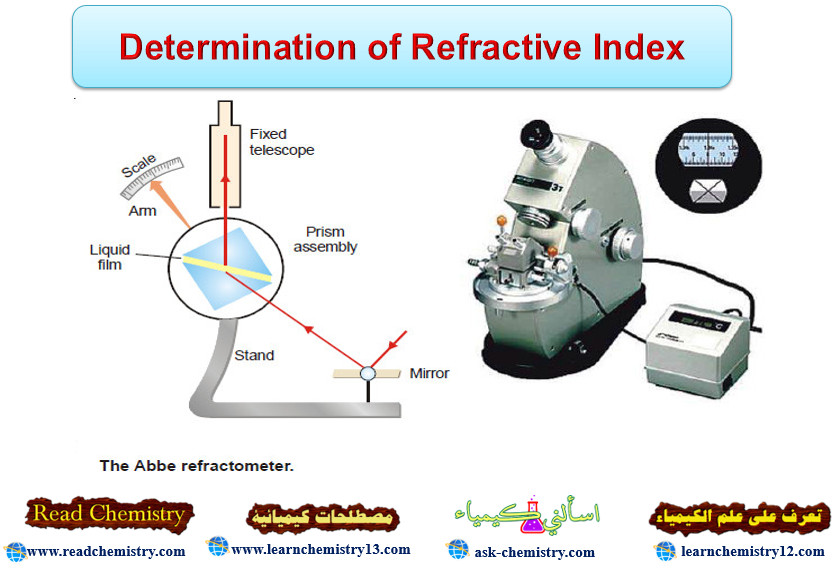
Determination of Refractive Index
Refractive Index – The refractive index (n) of a substance is defined as the ratio of the velocity of light…
Read More » -
Physical Chemistry
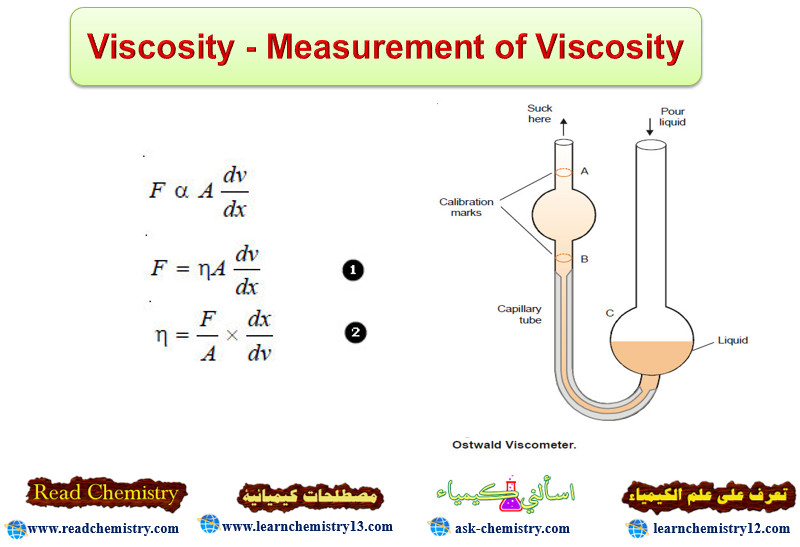
Viscosity – Measurement of Viscosity
Viscosity – Viscosity is the resistance of a liquid to flow. – A liquid may be considered to be consisting…
Read More » -
Physical Chemistry
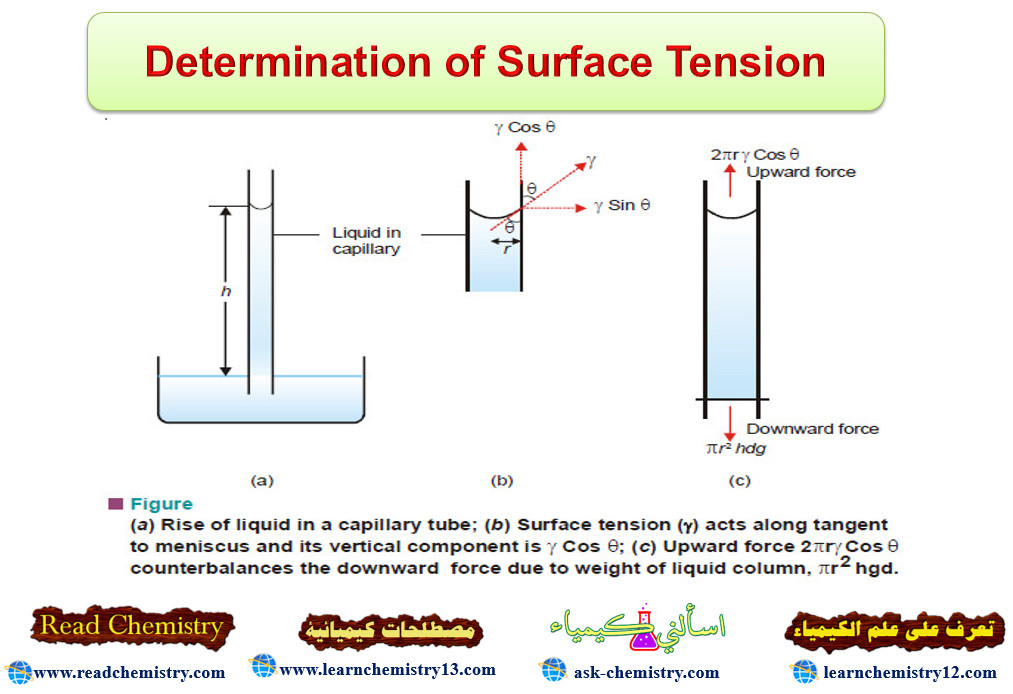
Determination of Surface Tension
Surface Tension – Surface Tension property of liquids arises from the intermolecular forces of attraction. – A molecule in the…
Read More » -
Physical Chemistry
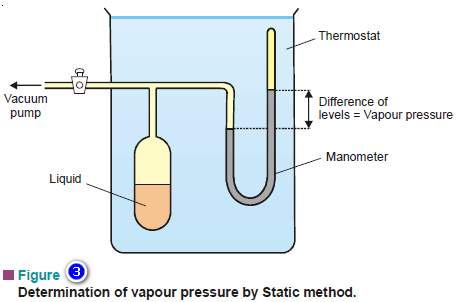
Vapour Pressure , Factors affecting on Vapour Pressure
– The vapour pressure of a liquid is defined as the pressure exerted by the vapour in equilibrium with the…
Read More » -
Physical Chemistry
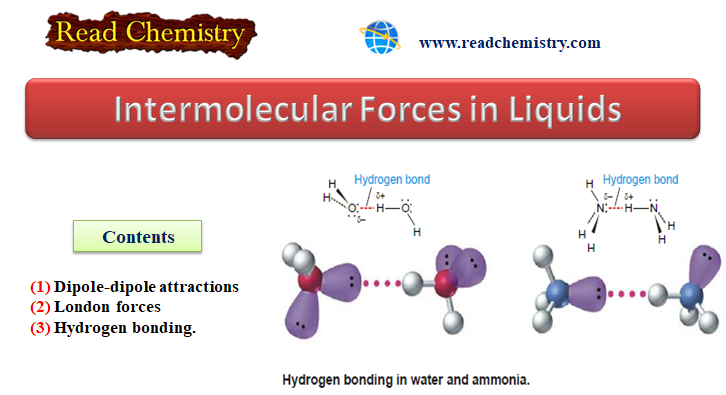
Intermolecular Forces in Liquids
Intermolecular Forces in Liquids – Intermolecular forces in liquids are collectively called van der Waals forces. – These forces are…
Read More » -
Physical Chemistry
Gases – General Characteristics of gases
States of the matter – All matter exists in three states: gases, liquids and solids. – A molecular-level representation of…
Read More » -
Physical Chemistry
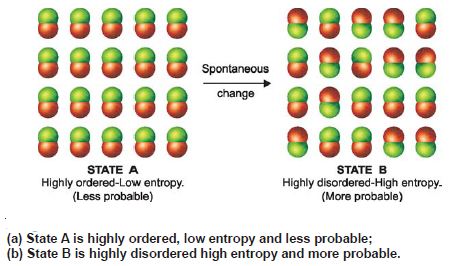
Spontaneous Processes – Second Law of Thermodynamics
Spontaneous Processes – A process that proceeds of its own accord, without any outside assistance, is termed a spontaneous or…
Read More » -
Physical Chemistry
MCQ on Chapter Thermochemistry ΔH, ΔE
1. For exothermic reactions, ΔH is _______ while for endothermic reactions it is _______. (a) positive, negative (b) positive, positive…
Read More » -
Physical Chemistry
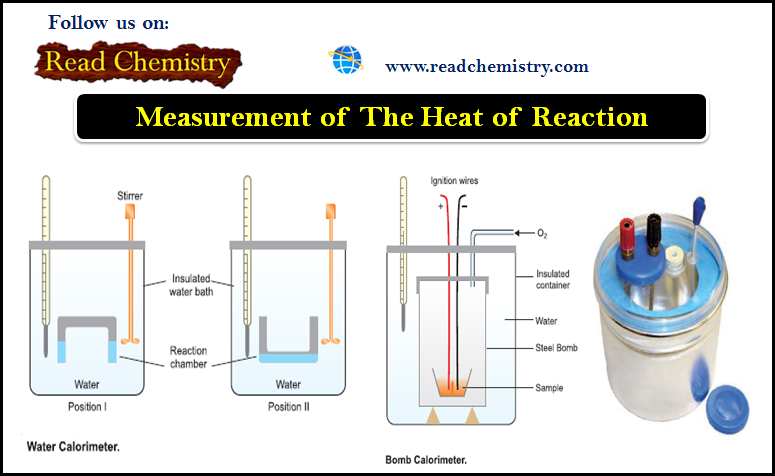
Measurement of The Heat of Reaction
Measurement of The Heat of Reaction – The experimental measurement of the heat of reaction or enthalpy change is known…
Read More »