-
General Chemistry
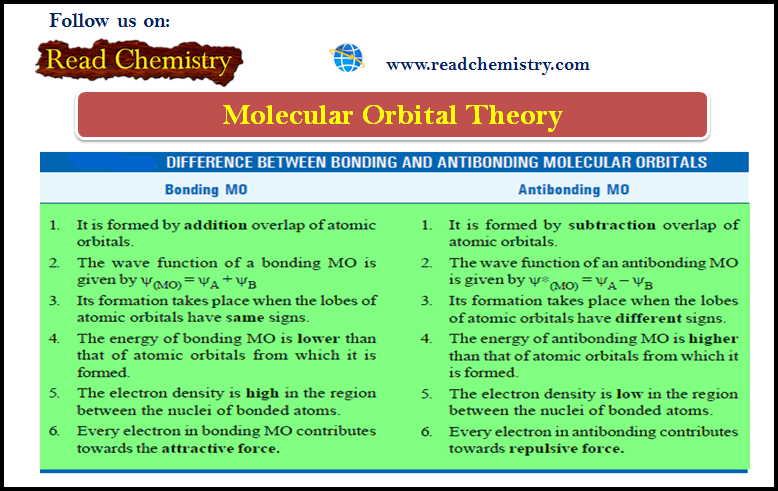
Molecular Orbital Theory
Molecular Orbital Theory – The molecular orbital theory proposed by Hund and Mulliken in 1932 explains the formation of a…
Read More » -
General Chemistry
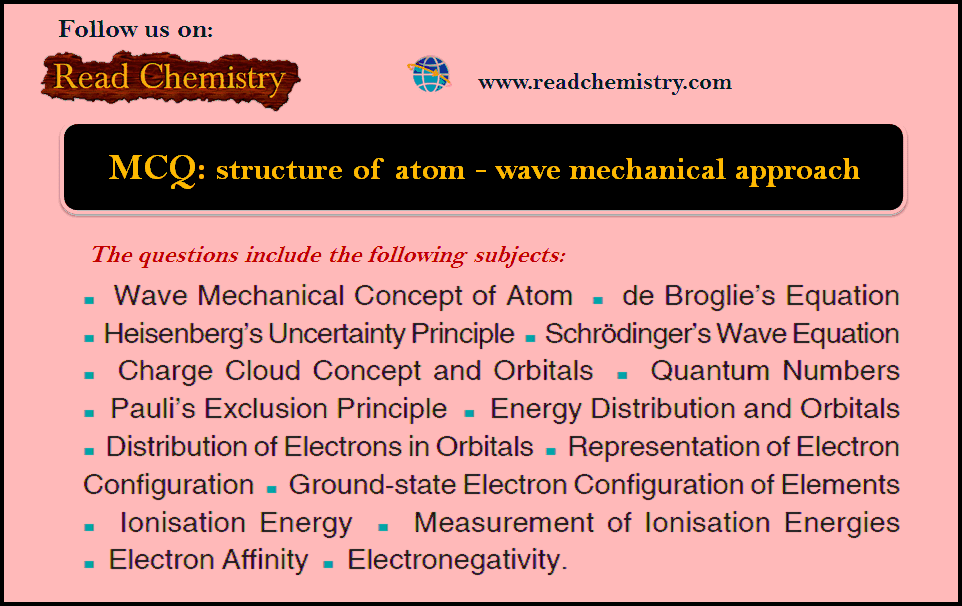
MCQ on Chapter: structure of atom – wave mechanical approach
MCQ on the structure of the atom – In this subject, you will find 50 questions and answers MCQ on…
Read More » -
General Chemistry
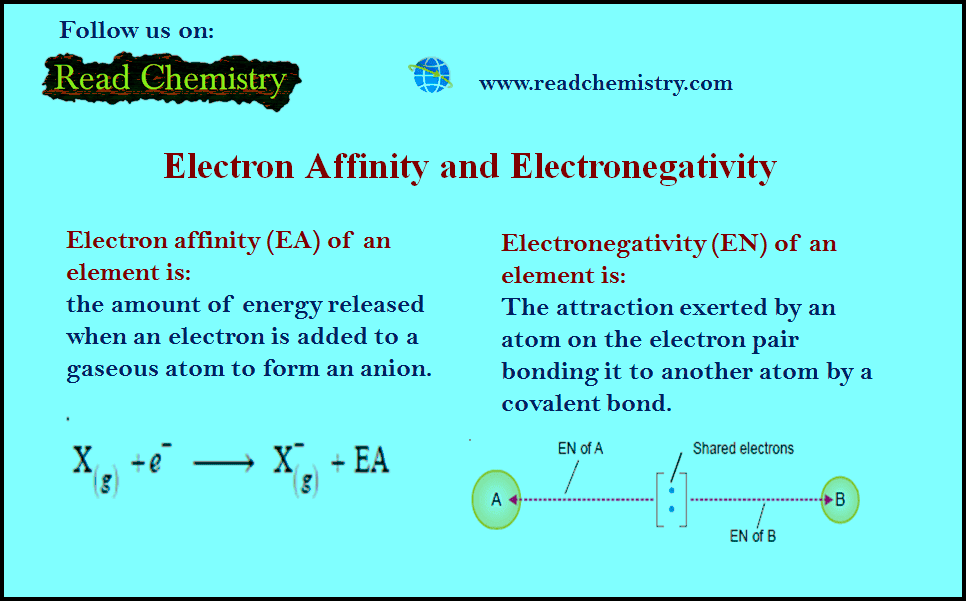
Electronegativity and Electron Affinity
– In this subject, we will discuss the difference Between Electronegativity and Electron Affinity Electron Affinity – A neutral atom…
Read More » -
General Chemistry
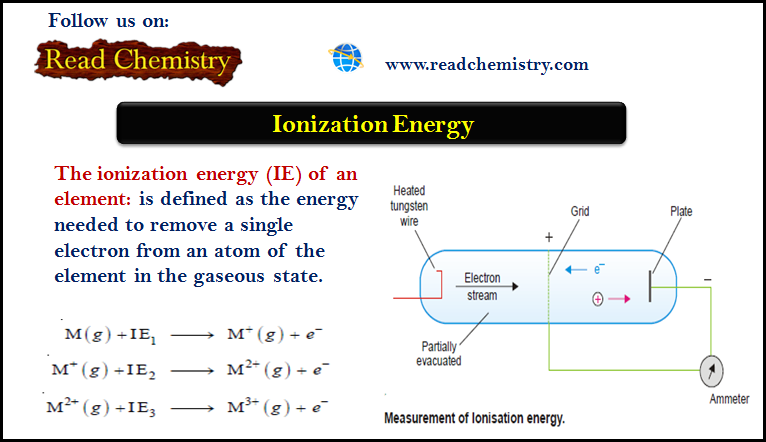
Ionization Energy (Definition – Trends – Measurement)
– The ionization energy (IE) of an element is defined as the energy needed to remove a single electron from…
Read More » -
General Chemistry
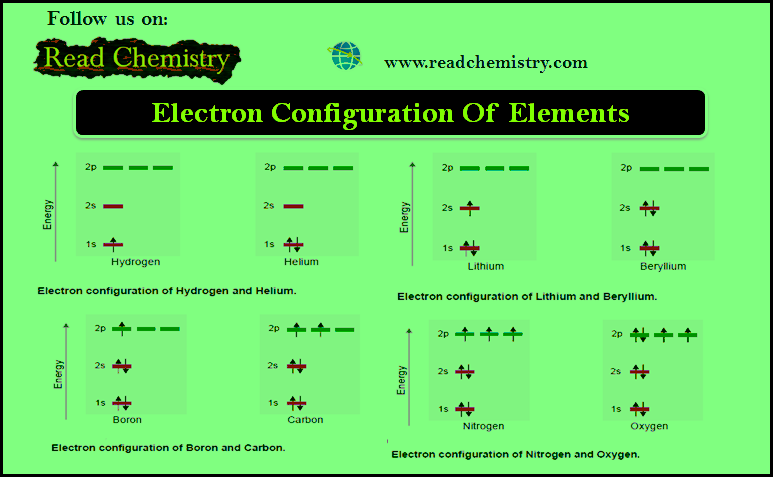
Electron Configuration Of Elements
– In this subject, we will discuss the rules of Electron Configuration Of Elements Electron Configuration Of Elements – We…
Read More » -
General Chemistry
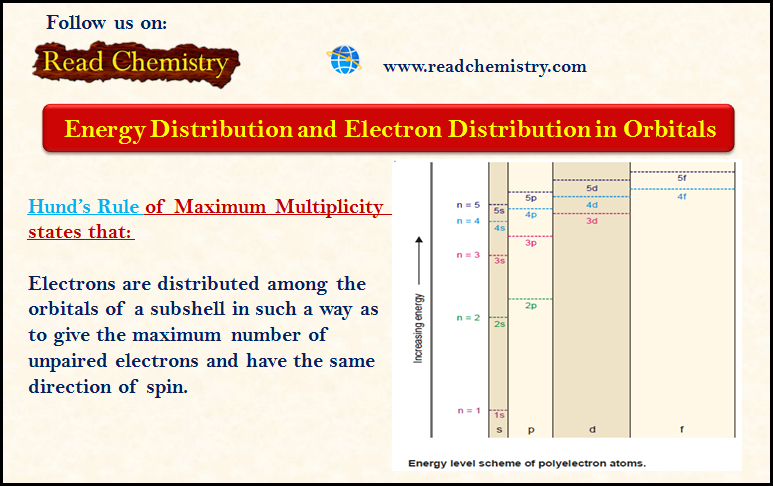
Distribution of Electrons in Orbitals
– In this subject, we will discuss the Distribution of Electrons in Orbitals according to Hund’s Rule. Energy Distribution and…
Read More » -
General Chemistry
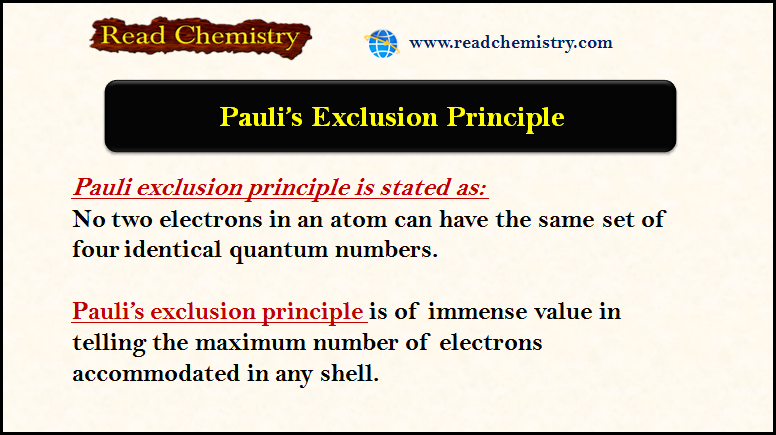
Pauli Exclusion Principle
– The Pauli exclusion principle is of immense value in telling the maximum number of electrons accommodated in any shell.…
Read More » -
General Chemistry
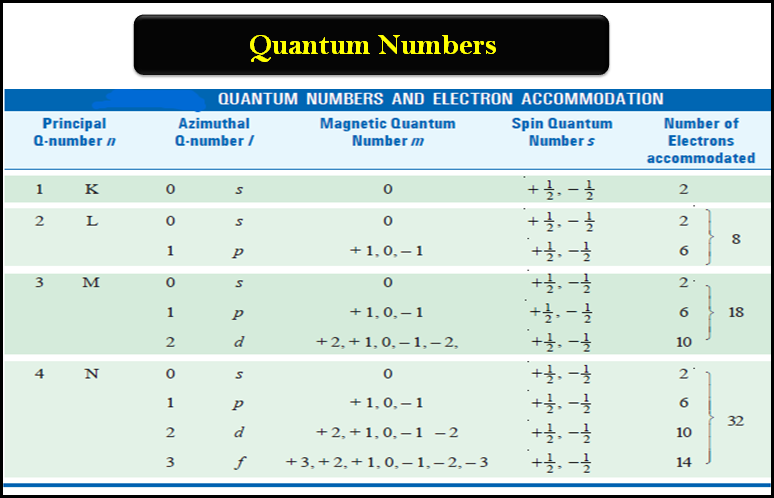
Quantum Numbers (Principal, Azimuthal, Magnetic and Spin)
Quantum Numbers – Bohr’s electronic energy shells or levels, designated as Principal Quantum Numbers (n), could hardly explain the hydrogen…
Read More » -
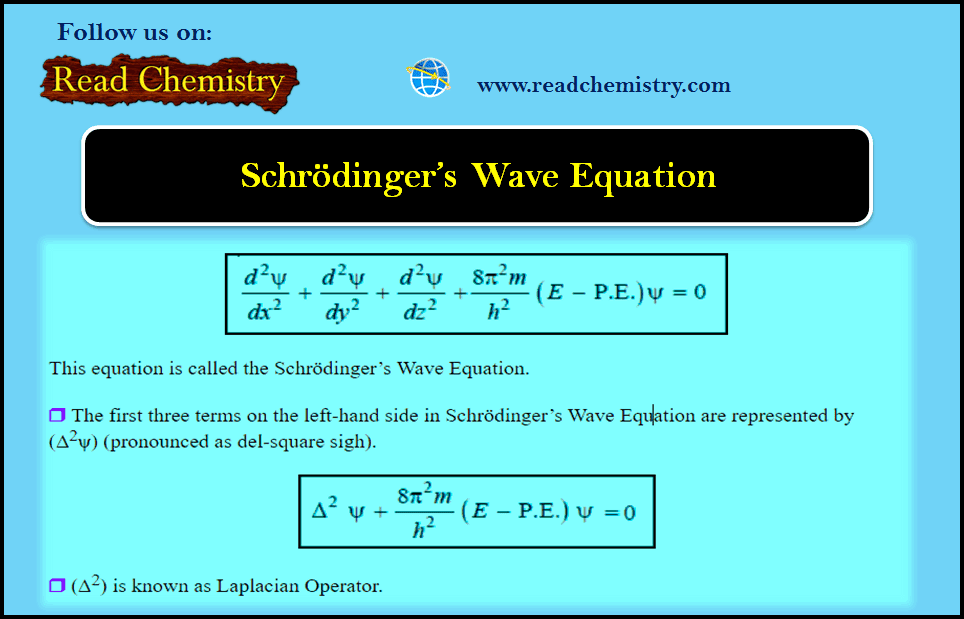
General Chemistry
Schrödinger Wave Equation
Schrödinger Wave Equation – In order to provide sense and meaning to the probability approach, Schrödinger derived an equation known…
Read More » -
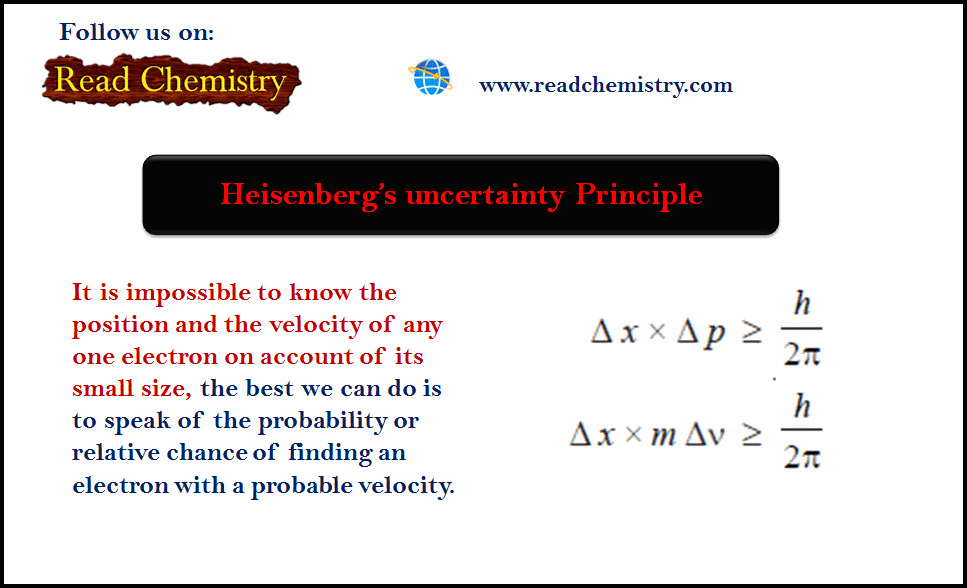
General Chemistry
Heisenberg’s uncertainty Principle
– Heisenberg’s uncertainty Principle: As it is impossible to know the position and the velocity of any one electron on…
Read More » -
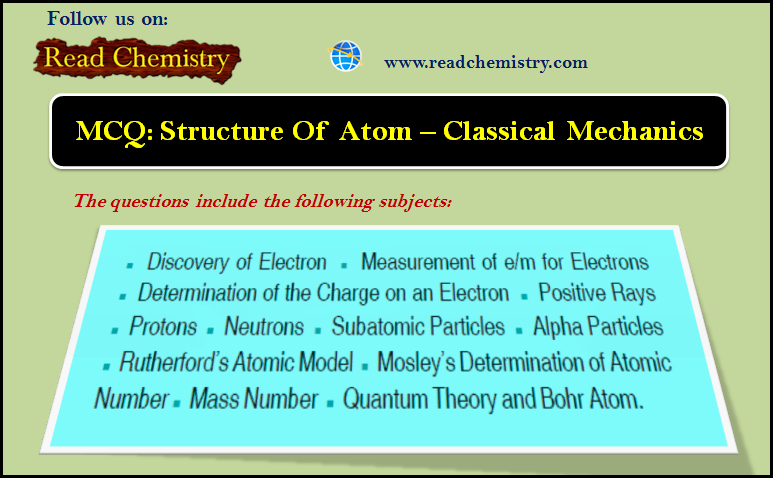
General Chemistry
MCQ: Structure of atom – Classical Mechanics
1. In the spectrum of hydrogen atom, the series which falls in ultraviolet region is_______ (a) Lyman series (b) Balmer…
Read More » -
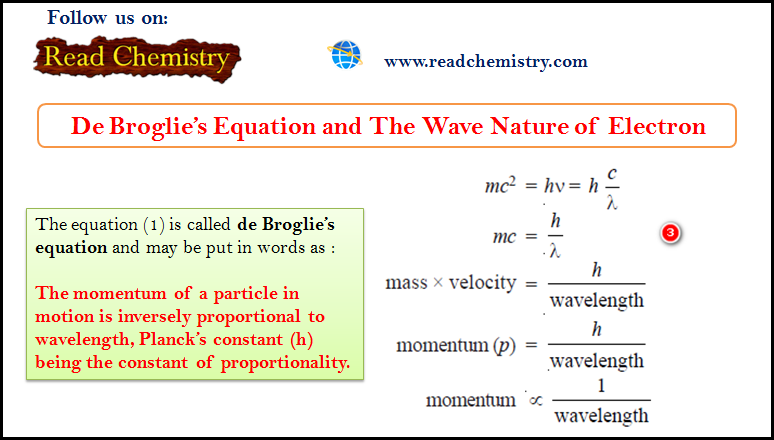
General Chemistry
De Broglie Equation – The Wave Nature of Electron
De Broglie Equation – de Broglie had arrived at his hypothesis (de Broglie equation) with the help of Planck’s Quantum…
Read More » -
General Chemistry
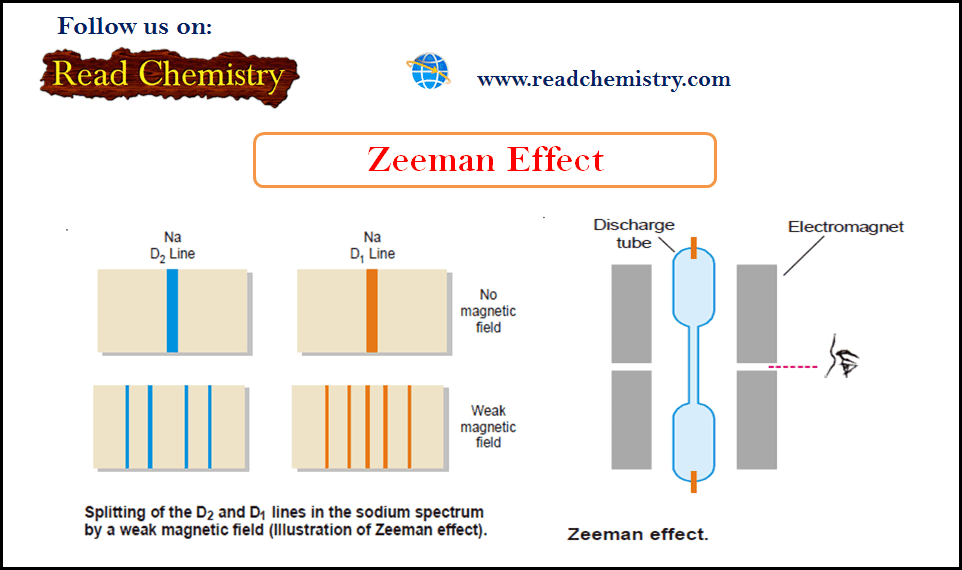
Zeeman Effect
Zeeman Effect – In 1896 Zeeman discovered that spectral lines are split up into components when the source emitting lines…
Read More » -
General Chemistry
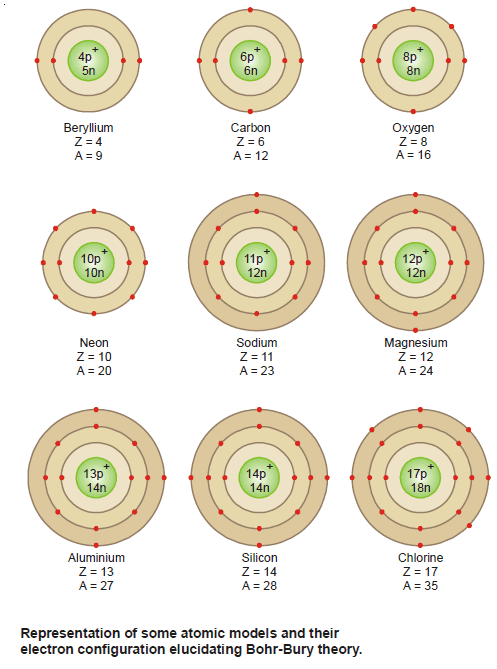
Bohr-Bury Scheme – Electron arrangement in the orbits
– The Bohr-Bury scheme considers that the maximum number of electrons that each orbit can contain is 2 × n2,…
Read More » -
General Chemistry
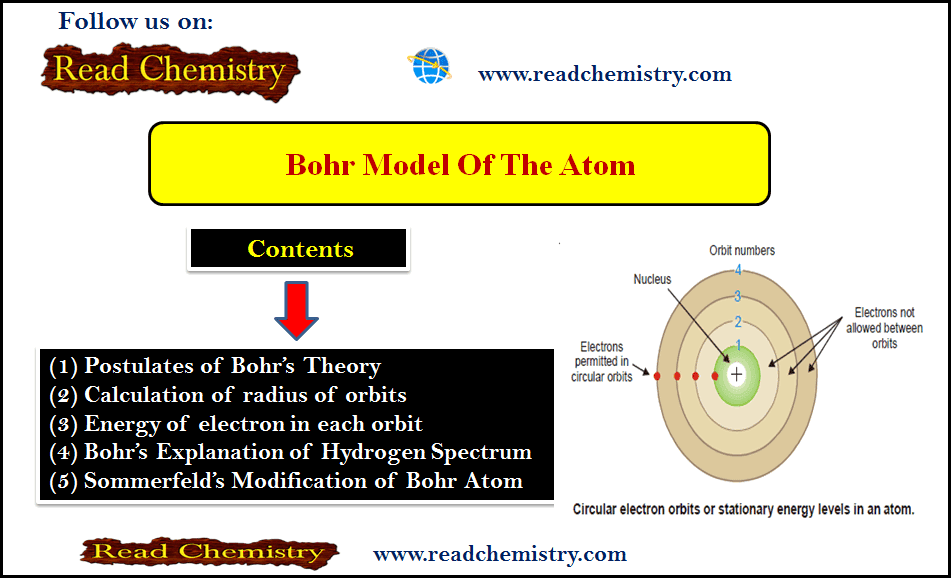
Bohr Model of atom – Bohr Theory
– Bohr theory was based on Planck’s quantum theory and was built on some postulates. – Rutherford’s nuclear model simply…
Read More » -
General Chemistry
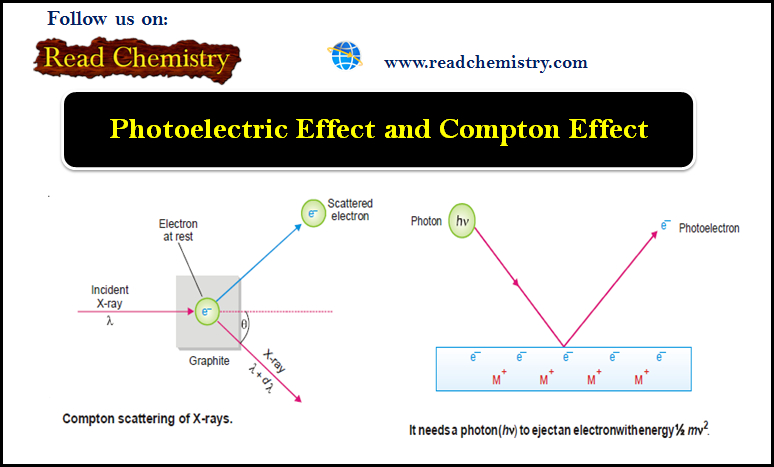
Photoelectric Effect and Compton Effect
– In this subject, we will discuss the Photoelectric Effect and Compton Effect Photoelectric Effect – When a beam of…
Read More » -
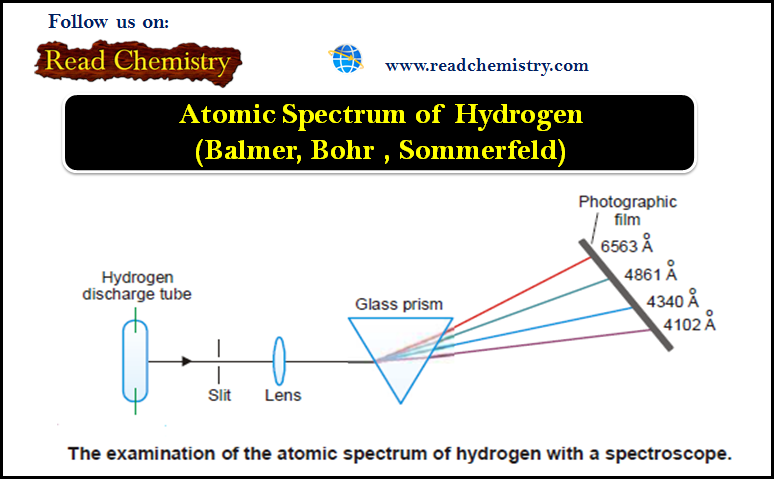
General Chemistry
Atomic Spectrum of Hydrogen
– In this subject, we will discuss the atomic Spectrum of Hydrogen Atomic Spectrum – When an element in the…
Read More » -
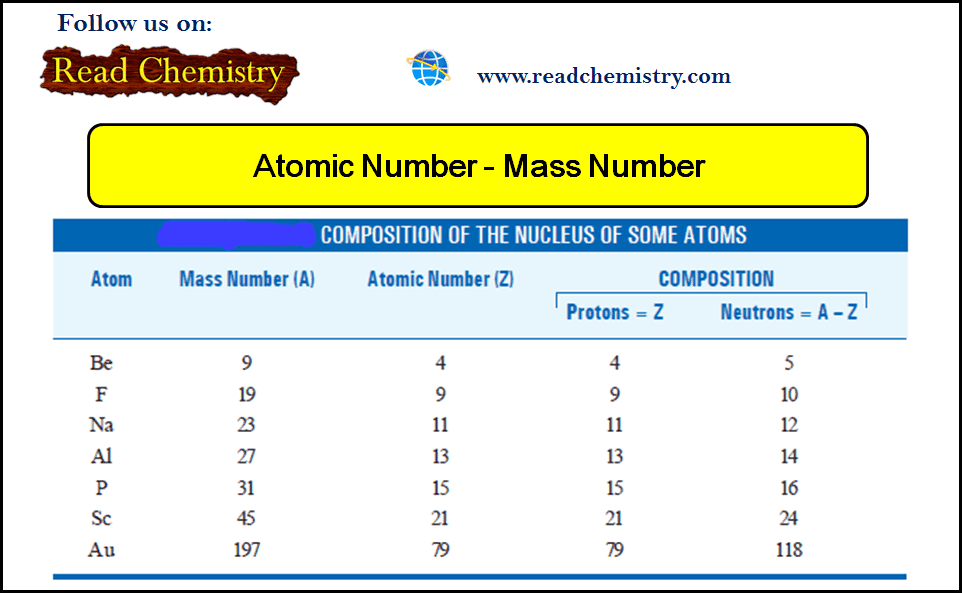
General Chemistry
Atomic Number and Mass Number
– In this subject, we will discuss the Atomic Number and Mass Number Mosley’s Determination of Atomic Number – The…
Read More » -
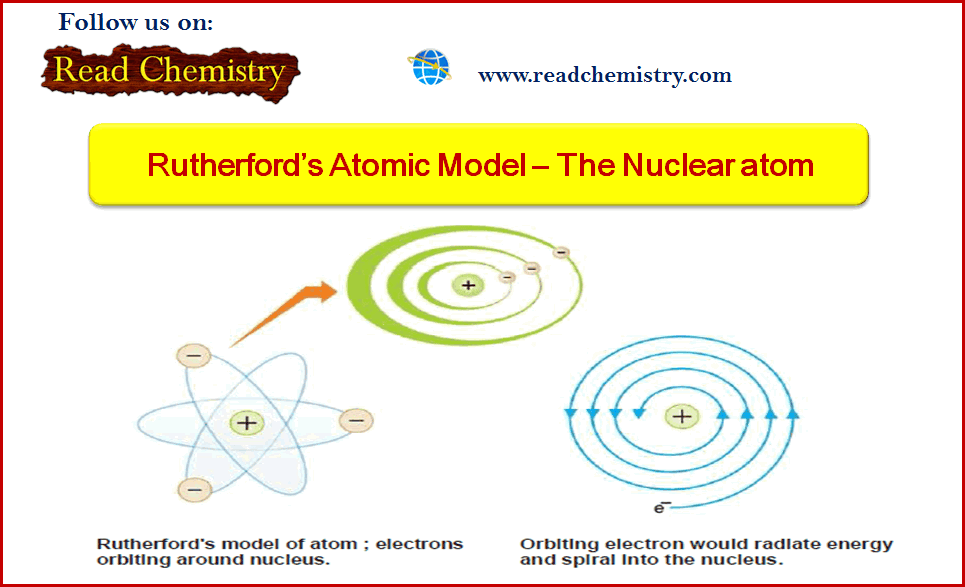
General Chemistry
Rutherford’s Atomic Model (Experiment, Postulates, weakness)
– In this subject, we will discuss the Rutherford’s Atomic Model (Experiment, Postulates, weakness) Alpha particles – Alpha particles are…
Read More » -
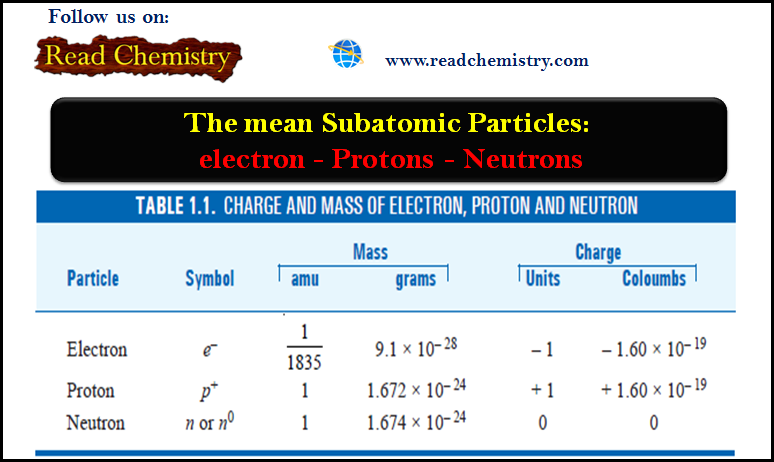
General Chemistry
Subatomic Particles: Electrons, Protons, and Neutrons
– In this subject, we will discuss the Subatomic Particles: Electrons, Protons, and Neutrons Subatomic Particles – We have hitherto…
Read More »