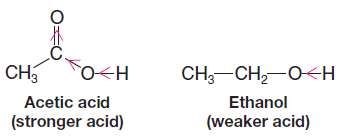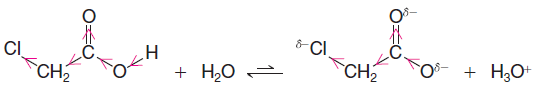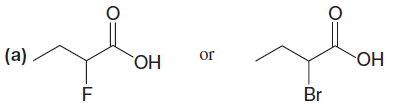Acidity of Carboxylic Acids and Alcohols
– In this subject, we will discuss the Acidity Differences between Alcohols and Carboxylic Acids
Acidity of Carboxylic Acids and Alcohols
– Carboxylic acids are weak acids, typically having pKa values in the range of 3–5.
– Alcohols, by comparison, have pKa values in the range of 15–18 and essentially do not give up a proton unless exposed to a very strong base.
– To understand the reasons for this difference, let’s consider acetic acid and ethanol as representative examples of simple carboxylic acids and alcohols.
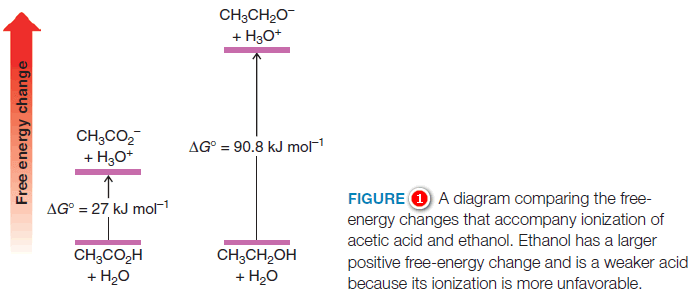
– Using the pKa for acetic acid (4.75), one can calculate that the free energy change (∆Go) for ionization of the carboxyl proton of acetic acid is +27 kJ mol-1, a moderately endergonic (unfavorable) process since the (∆Go) value is positive.
– Using the pKa of ethanol (16), one can calculate that the corresponding free-energy change for ionization of the hydroxyl proton of ethanol is +90.8 kJ mol-1, a much more endergonic (and hence even less favorable) process.
– These calculations reflect the fact that ethanol is much less acidic than acetic acid.
– Figure (1) depicts the magnitude of these energy changes in a relative sense.
– How do we explain the much greater acidity of carboxylic acids than alcohols?
The acidity of carboxylic acids is much greater than alcohols
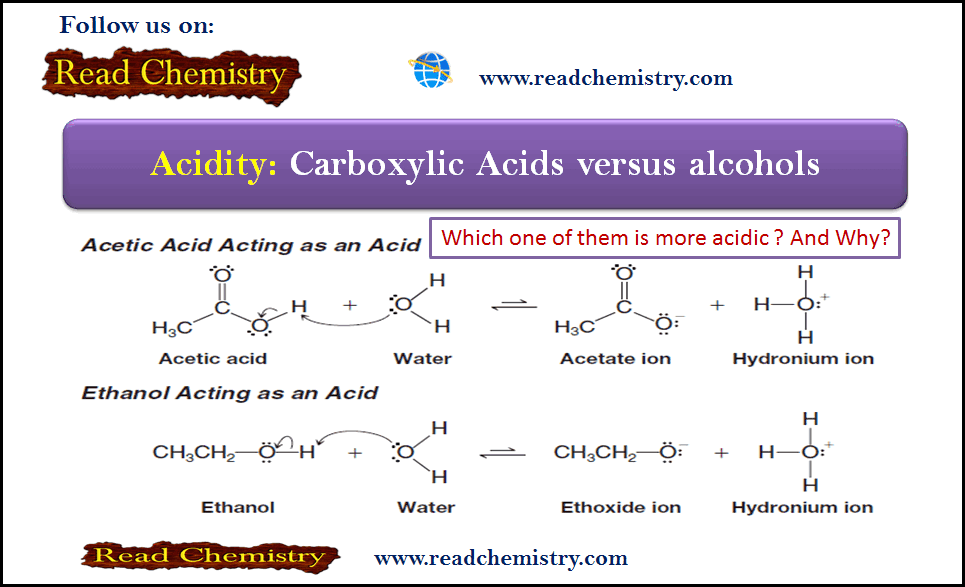
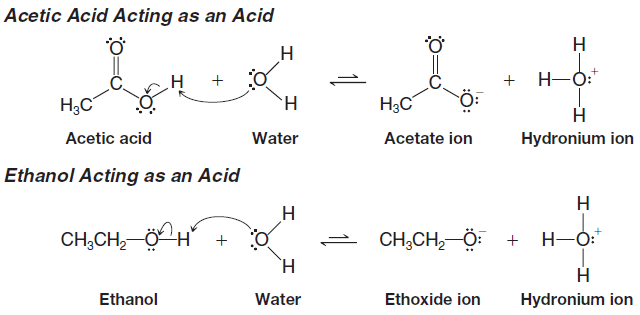
– Consider first the structural changes that occur if both acetic acid and ethanol act as acids by donating a proton to water.
– What we need to focus on is the relative stability of the conjugate bases derived from a carboxylic acid and an alcohol.
– This is because the smaller free-energy change for ionization of a carboxylic acid (e.g., acetic acid) as compared to an alcohol (e.g., ethanol) has been attributed to greater stabilization of the negative charge in the carboxylate ion as compared to an alkoxide ion.
– Greater stabilization of the carboxylate ion appears to arise from two factors:
(a) Delocalization of charge (as depicted by resonance structures for the carboxylate ion).
(b) An inductive electron-withdrawing effect
(1) The Effect of Delocalization
– Delocalization of the negative charge is possible in a carboxylate anion, but it is not possible in an alkoxide ion.
– We can show how delocalization is possible in carboxylate ions by writing resonance structures for the acetate ion.
– The two resonance structures we drew above distributed the negative charge to both oxygen atoms of the carboxylate group, thereby stabilizing the charge.
– This is a delocalization effect (by resonance).
– In contrast, no resonance structures are possible for an alkoxide ion, such as ethoxide.
– A rule to keep in mind is that charge delocalization is always a stabilizing factor, and because of charge stabilization, the energy difference for formation of a carboxylate ion from a carboxylic acid is less than the energy difference for formation of an alkoxide ion from an alcohol.
– Since the energy difference for ionization of a carboxylic acid is less than for an alcohol, the carboxylic acid is a stronger acid.
(2) The Inductive Effect
– We have already shown how the negative charge in a carboxylate ion can be delocalized over two oxygen atoms by resonance.
– However, the electronegativity of these oxygen atoms further helps to stabilize the charge, by what is called an inductive electron-withdrawing effect.
– A carboxylate ion has two oxygen atoms whose combined electronegativity stabilizes the charge more than in an alkoxide ion, which has only a single electronegative oxygen atom.
– In turn, this lowers the energy barrier to forming the carboxylate ion, making a carboxylic acid a stronger acid than an alcohol.
– This effect is evident in electrostatic potential maps depicting approximately the bonding electron density for the two anions (Figure (2).
– The negative charge in the acetate anion is evenly distributed over the two oxygen atoms, whereas in ethoxide the negative charge is localized on its sole oxygen atom (as indicated by red in the electrostatic potential map).
– It is also reasonable to expect that a carboxylic acid would be a stronger acid than an alcohol when considering each as a neutral molecule (i.e., prior to loss of a proton), because both functional groups have a highly polarized O-H bond, which in turn weakens the bond to the hydrogen atom.
– However, the significant electron-withdrawing effect of the carbonyl group in acetic acid and the absence of an adjacent electron-withdrawing group in ethanol make the carboxylic acid hydrogen much more acidic than the alcohol hydrogen.
– Electrostatic potential maps at approximately the bond density surface for acetic acid and ethanol (Figure (3) clearly show the positive charge at the carbonyl carbon of acetic acid, as compared to the CH2 carbon of ethanol.
(3) Summary and a Comparison of Conjugate Acid–Base Strengths
– In summary, the greater acidity of a carboxylic acid is predominantly due to the ability of its conjugate base (a carboxylate ion) to stabilize a negative charge better than an alkoxide ion, the conjugate base of an alcohol.
– In other words, the conjugate base of a carboxylic acid is a weaker base than the conjugate base of an alcohol.
– Therefore, since there is an inverse strength relationship between an acid and its conjugate base, a carboxylic acid is a stronger acid than an alcohol.
(4) Inductive Effects of Other Groups
– The acid-strengthening effect of other electron-attracting groups (other than the carbonyl group) can be shown by comparing the acidities of acetic acid and chloroacetic acid:
– This is an example of a substituent effect.
– The greater acidity of chloroacetic acid can be attributed, in part, to the extra electron-attracting inductive effect of the electronegative chlorine atom.
– By adding its inductive effect to that of the carbonyl group and the oxygen, it makes the hydroxyl proton of chloroacetic acid even more positive than that of acetic acid.
– It also stabilizes the chloroacetate ion that is formed when the proton is lost by dispersing its negative charge (Figure (4):
– Dispersal of charge always makes a species more stable, and, as we have seen now in several instances, any factor that stabilizes the conjugate base of an acid increases the strength of the acid.
Solved Problems on Acidity of Carboxylic Acids and Alcohols
Which compound in each pair would be most acidic?
Strategy and Answer:
– Decide what is similar in each pair and what is different.
In pair (a):
– The difference is the halogen substituent on the carbon adjacent to the carboxyl group.
– In the first example it is fluorine; in the second it is bromine.
– Fluorine is much more electronegative (electron-attracting) than bromine, therefore it will be able to disperse the negative charge of the anion formed when the proton is lost.
– Thus the first compound will be the stronger acid.
In pair (b):
– The difference is the position of the fluorine substituents.
– In the second compound the fluorine is closer to the carboxyl group where it will be better able to disperse the negative charge in the anion formed when the proton is lost.
– Thus The second compound will be the stronger acid.
Reference: Organic chemistry / T.W. Graham Solomons, Craig B.Fryhle, Scott A. Snyder, / ( eleventh edition) / 2014.