-
Organic Chemistry
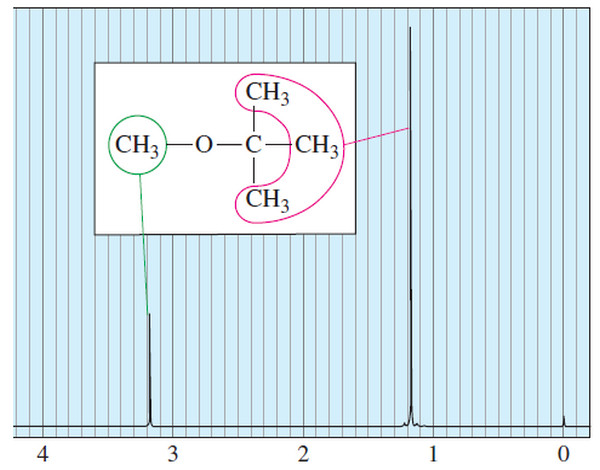
Number of Signals in NMR Spectroscopy
The Number of Signals – In general, the number of NMR signals corresponds to the number of different kinds of…
Read More » -
Physical Chemistry
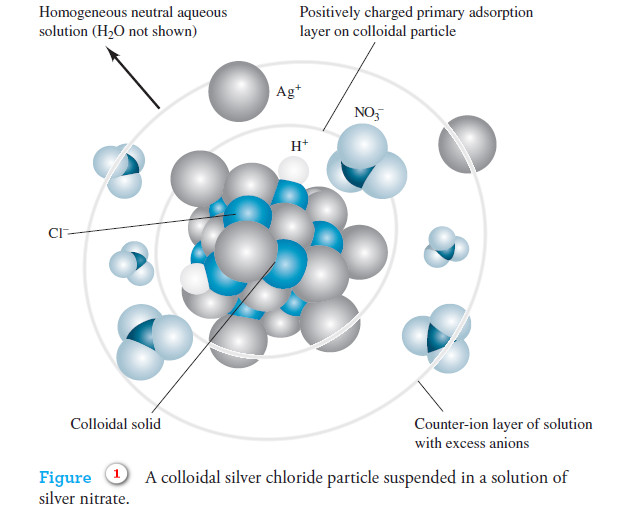
Colloids: Definition, History and Types
– In this topic, we will discuss the colloids: definition, History and Types History of colloids – Thomas Graham (1861)…
Read More » -
Analytical Chemistry
Some Terms Used in Volumetric Titration
Some Terms Used in Volumetric Titration – A standard solution (or a standard titrant) is a reagent of known concentration…
Read More » -
Analytical Chemistry
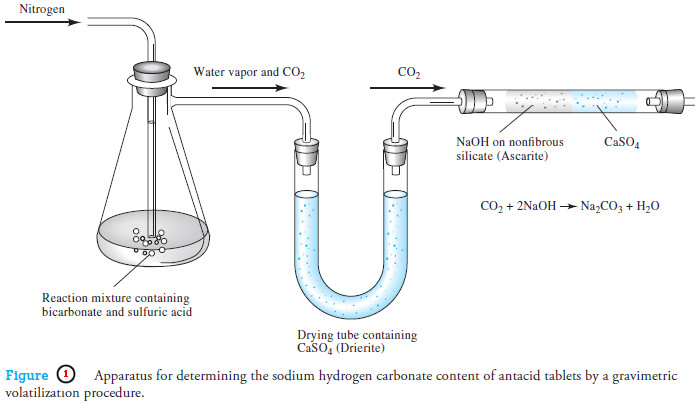
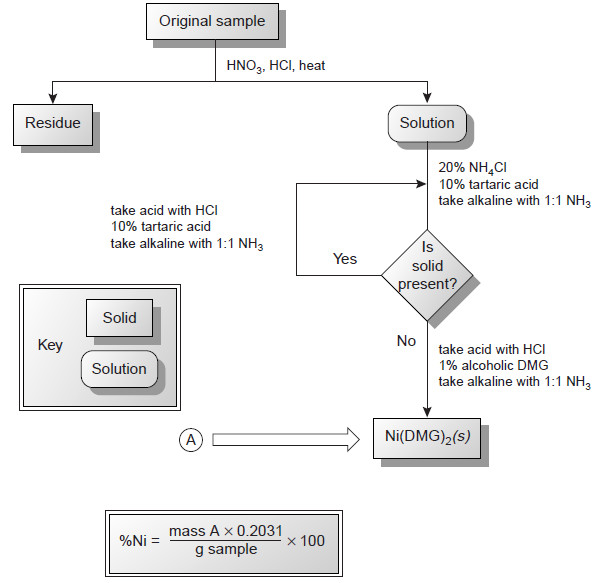
Applications of Gravimetric methods
Applications of Gravimetric methods – Gravimetric methods have been developed for most inorganic anions and cations, as well as for…
Read More » -
Organic Chemistry
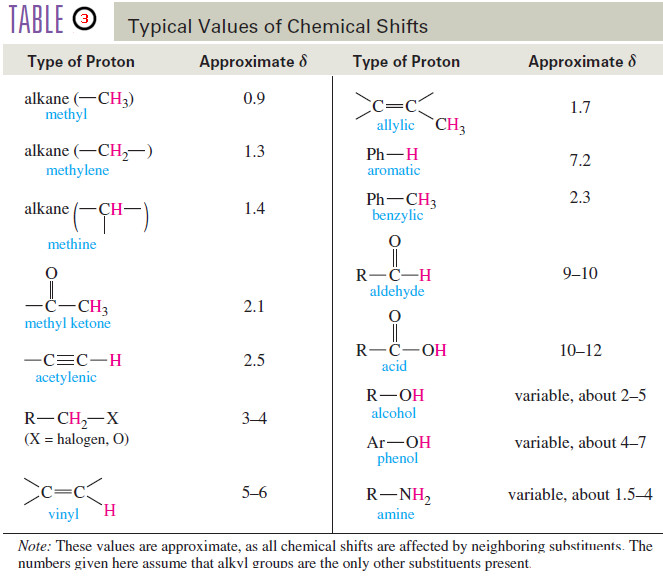
Chemical Shift in NMR Spectroscopy
– In this topic, we will discuss the Chemical Shift in 1H NMR Spectroscopy. What is Chemical Shift? – The…
Read More » -
Analytical Chemistry
Precipitation Gravimetry
– In this topic, we will discuss the Precipitation Gravimetry as an important one of Gravimetric Analysis in analytical chemistry.…
Read More » -
Analytical Chemistry
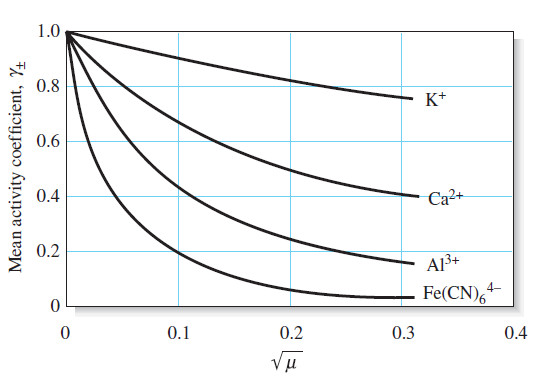
Activity Coefficients : Definition, Equation, Examples, Properties
– In this topic, we will discuss Activity coefficients : Definition, Equation, Examples and Properties. Activity Coefficients – Chemists use…
Read More » -
Analytical Chemistry
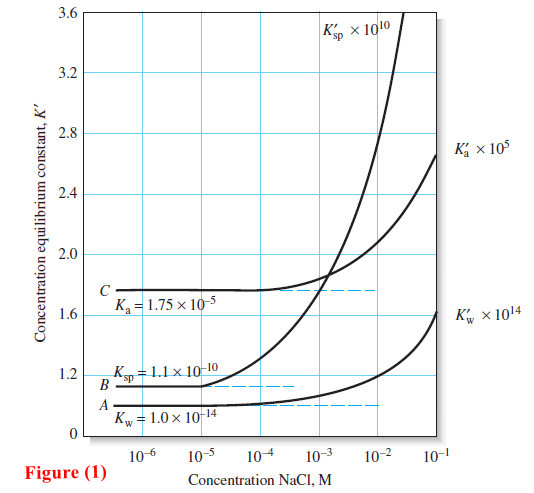
The Effect of Electrolyte on Chemical Equilibria
The Effect of Electrolyte on Chemical Equilibria – Experimentally, we find that the position of most solution equilibria depends on…
Read More » -
Analytical Chemistry
What is Analytical Chemistry?
What is Analytical Chemistry? Analytical chemistry is what analytical chemists do. – Analytical chemistry is too broad and active a…
Read More » -
Physical Chemistry
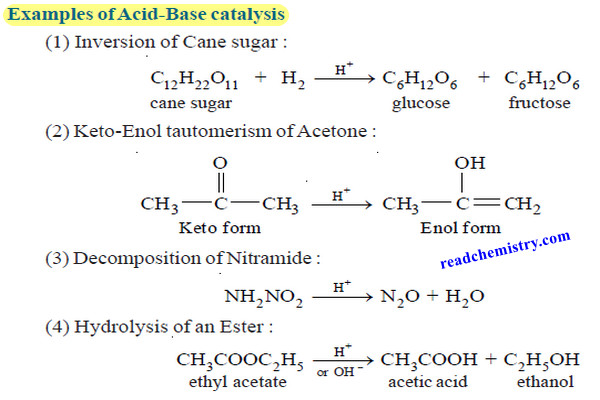
Acid-Base catalysis (definition – Examples – Mechanism)
– In this topic, we will discuss Acid-Base catalysis: definition, Examples and Mechanism. Acid-Base catalysis – A number of homogeneous…
Read More » -
Organic Chemistry
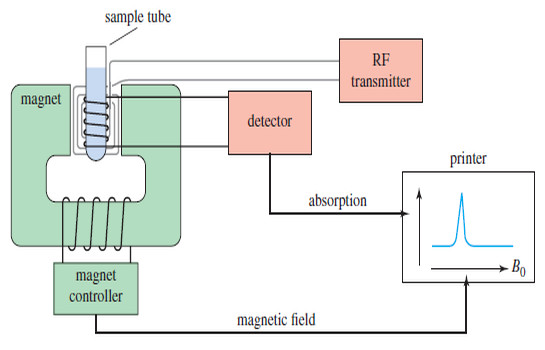
NMR spectrometer
What happens in an NMR spectrometer? – Before discussing the design of spectrometers, let’s review what happens in an NMR…
Read More » -
Physical Chemistry
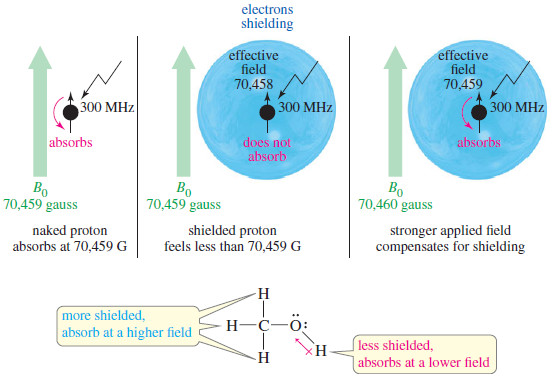
Magnetic Shielding by Electrons
– In this topic, we will discuss the Magnetic Shielding by Electrons Magnetic Shielding by Electrons – Up to now,…
Read More » -
Physical Chemistry
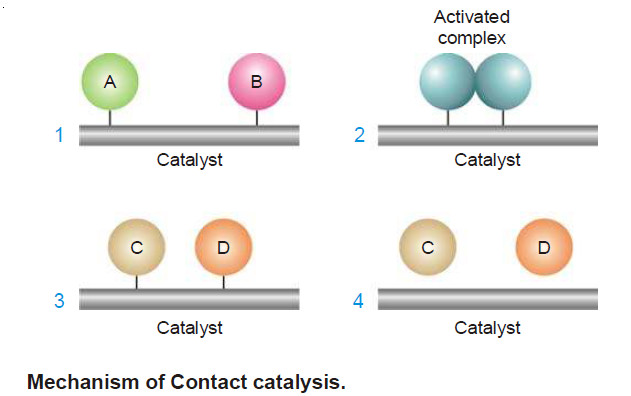
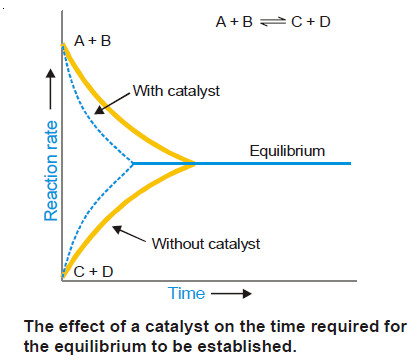
Theories of catalysis
Theories of Catalysis – There are two main theories of catalysis: (1) Intermediate Compound Formation theory. (2) The Adsorption theory.…
Read More » -
Physical Chemistry
Autocatalysis, Catalytic poisoning and Negative Catalysis
– In this topic, we will discuss Autocatalysis, Catalytic poisoning and Negative Catalysis. Catalytic poisoning – Very often a heterogeneous…
Read More » -
Physical Chemistry
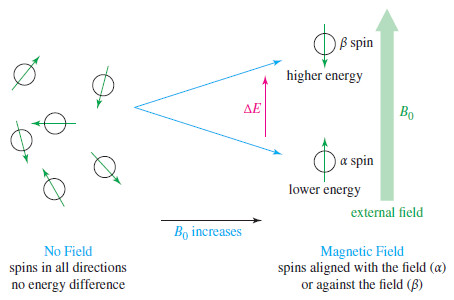
NMR – Theory of Magnetic Nuclear Resonance
Introduction to NMR – Nuclear magnetic resonance spectroscopy (NMR) is the most powerful tool available for organic structure determination. –…
Read More » -
Physical Chemistry
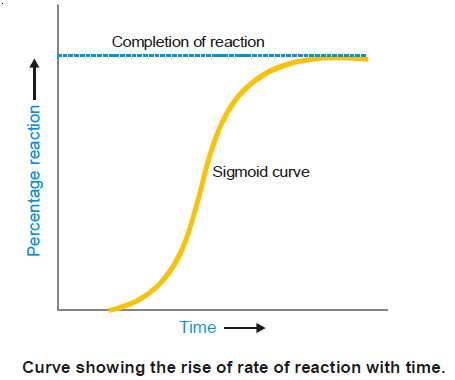
Characteristics of Catalytic Reactions
– In this topic, we will discuss general Characteristics of Catalytic Reactions and Promoters. General Characteristics of Catalytic Reactions –…
Read More » -
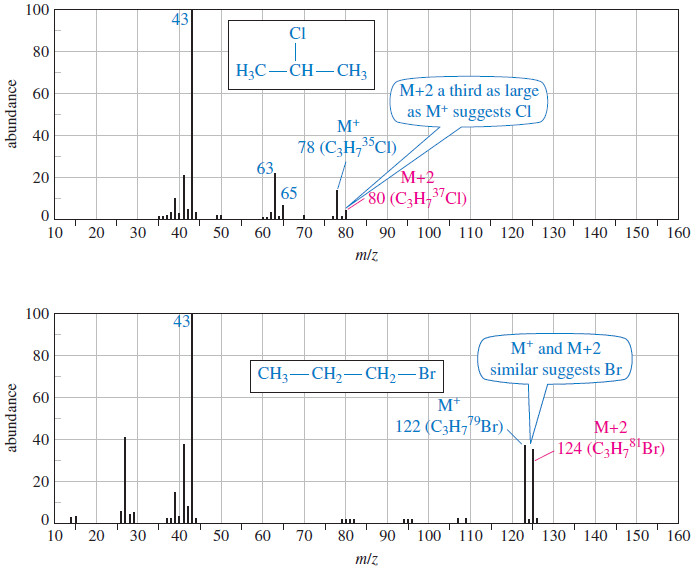
Organic Chemistry
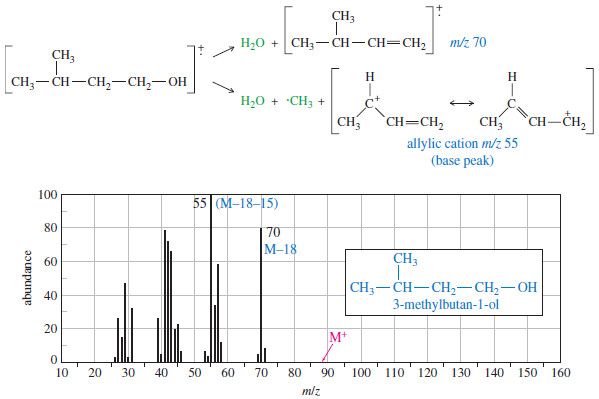
Fragmentation Patterns in Mass Spectrometry
– In this topic, we will discuss the Fragmentation Patterns in Mass Spectrometry. Fragmentation Patterns in Mass Spectrometry – In…
Read More » -
Physical Chemistry
Catalysis: Types of Catalysis
– In this topic, we will discuss the Catalysis and The types of catalysis : Homogeneous catalysis – Heterogenous catalysis.…
Read More » -
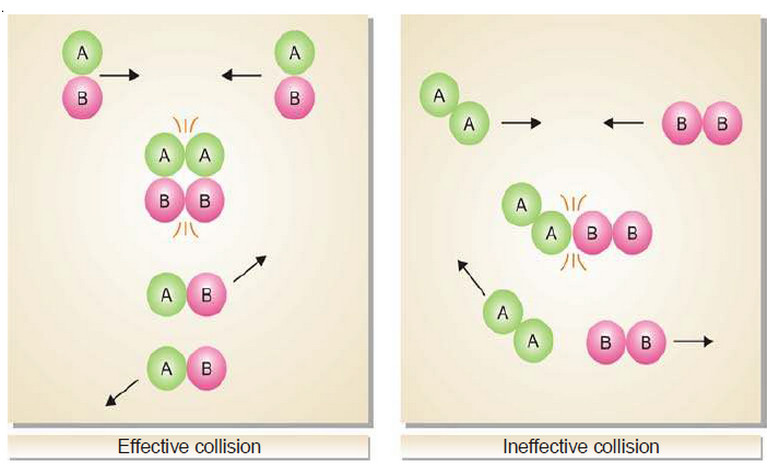
Physical Chemistry
Collision theory of Reaction rates
Collision theory of Reaction rates – According to collision theory, a chemical reaction takes place only by collisions between the…
Read More » -
Organic Chemistry
Determination of the Molecular Formula by Mass Spectrometry
Determination of the Molecular Formula by Mass Spectrometry – we can Determine the Molecular Formula by Mass Spectrometry and we…
Read More »