-
Analytical Chemistry
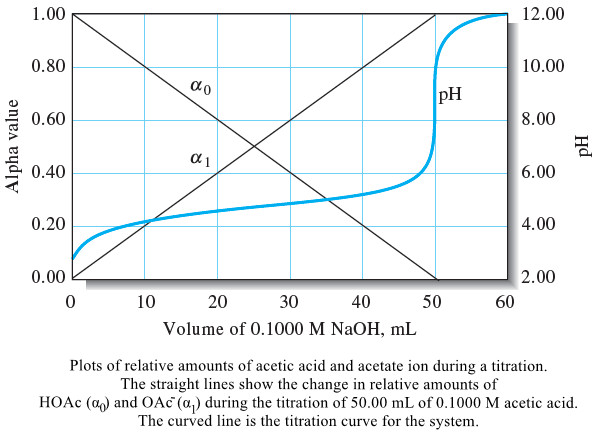
The Composition of Solutions During acid/Base Titration
– In this topic, we will discuss The Composition of Solutions During acid/Base Titration. The Composition of Solutions During acid/Base…
Read More » -
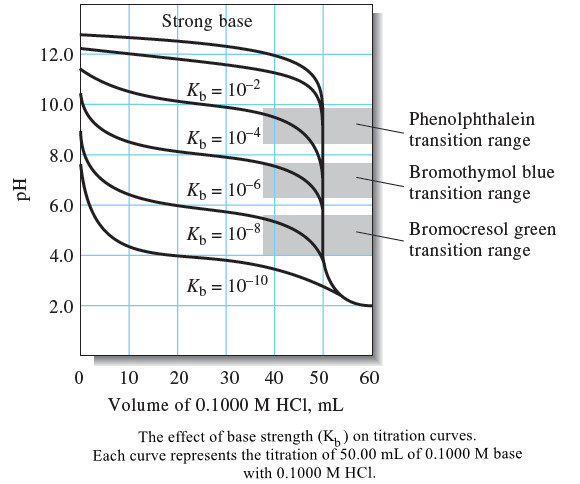
Analytical Chemistry
Titration Curves for Weak Bases
– In this topic, we will discuss The titration Curves for Weak Bases. Titration Curves for Weak Bases – The…
Read More » -
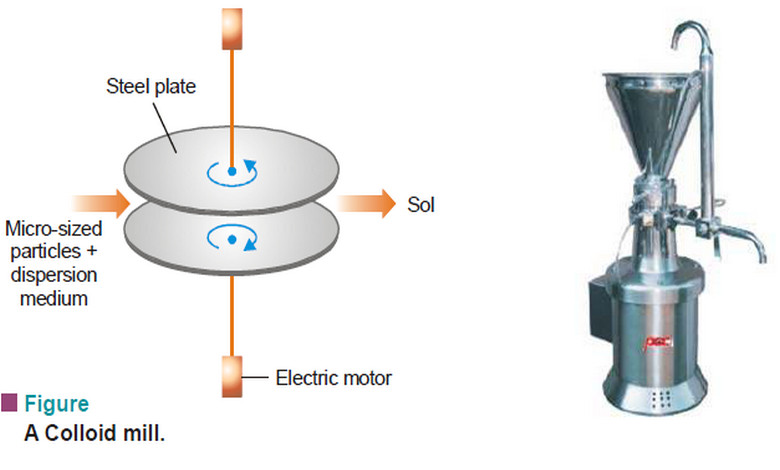
Physical Chemistry
Preparation of Sols and Purification of Sols
– In this topic, we will discuss Preparation of Sols, Purification of Sols and its colors Preparation of Sols –…
Read More » -
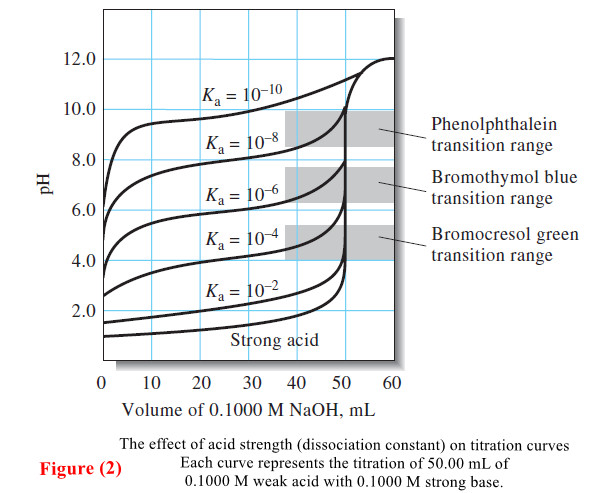
Analytical Chemistry
Titration Curves for Weak Acids
Titration Curves for Weak Acids – Four distinctly different types of calculations are needed to compute values for a weak…
Read More » -
Organic Chemistry
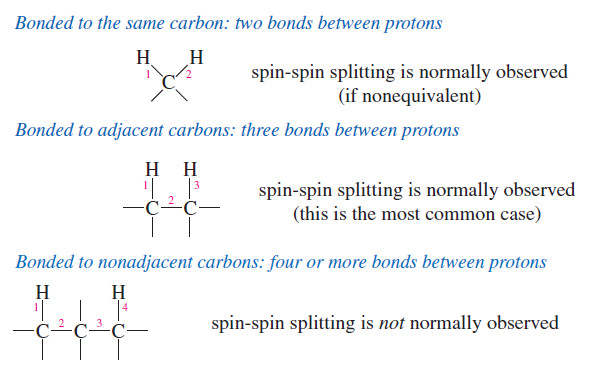
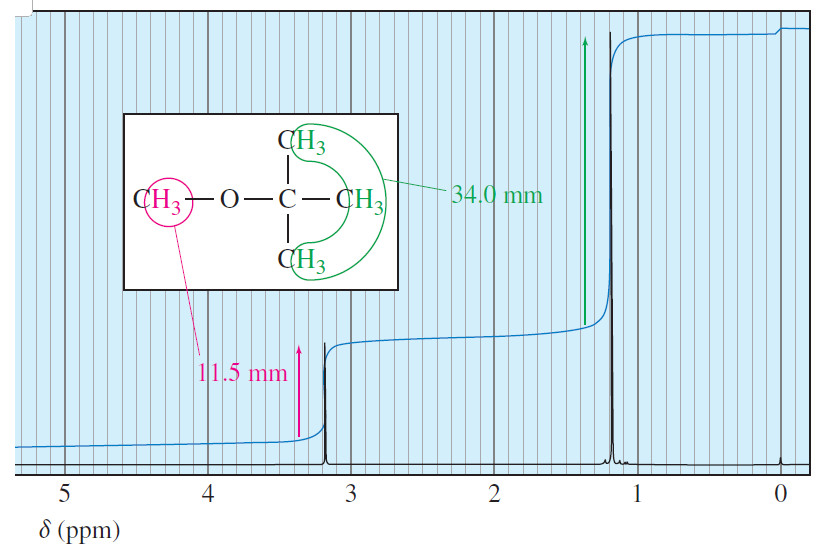
Spin-Spin Splitting in ¹H NMR Spectra
– In this topic, we will discuss The Spin-Spin Splitting in ¹H NMR Spectra. Theory of Spin-Spin Splitting – A…
Read More » -
Physical Chemistry
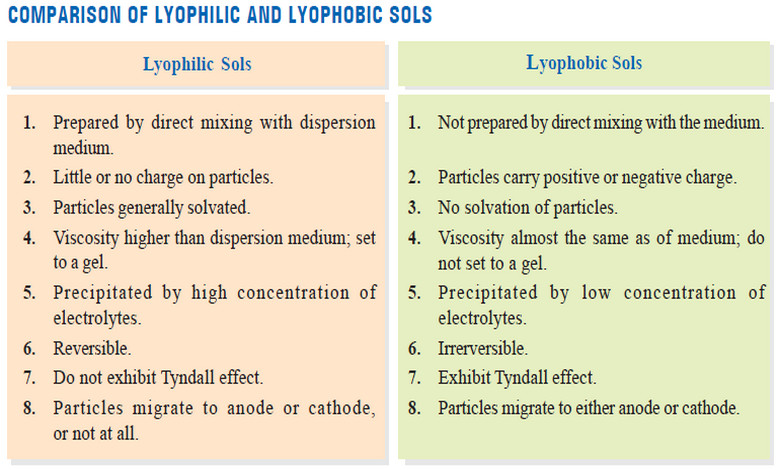
Lyophilic and Lyophobic sols : Defination, Properties, Comparison
– In this topic, we will discuss The Lyophilic and Lyophobic sols : Defination, Characteristics, and Comparison. Defination of Lyophilic…
Read More » -
Analytical Chemistry
Titration of Strong Acids and Bases
– In this topic, we will discuss The Titration of Strong Acids and Bases. Introduction to Titration of Strong Acids…
Read More » -
Analytical Chemistry
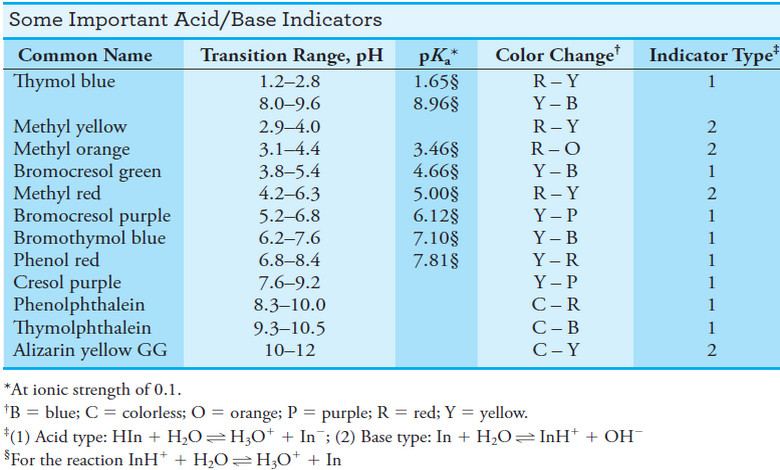
Indicators and Solutions for acid-base titration
– In this topic, we will discuss the Indicators and Solutions for acid-base titration. Indicators and Solutions for acid-base titration…
Read More » -
Analytical Chemistry
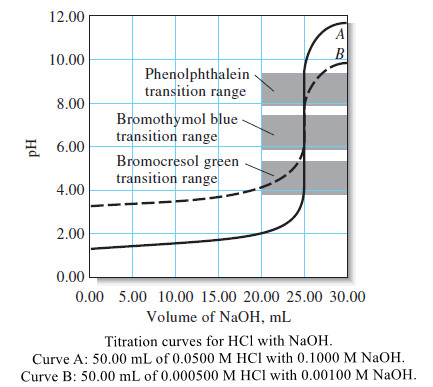
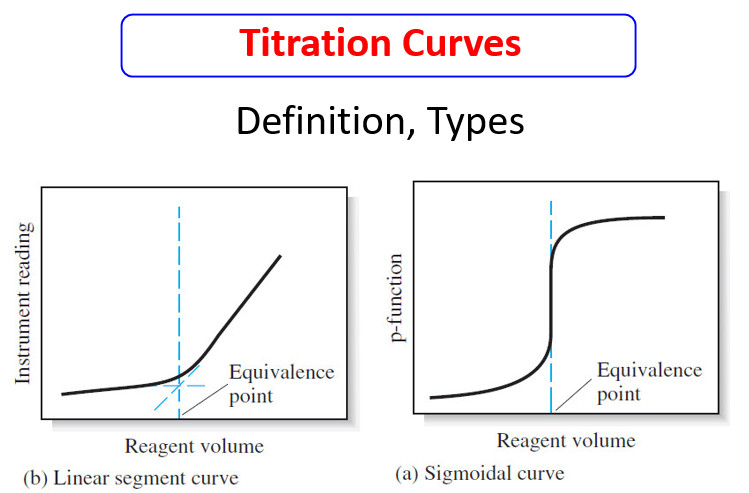
Titration Curves in Analytical Chemistry : Definition, Types
– In this topic, we will discuss the Titration Curves in Analytical Chemistry : Definition and Types Titration Curves –…
Read More » -
Analytical Chemistry
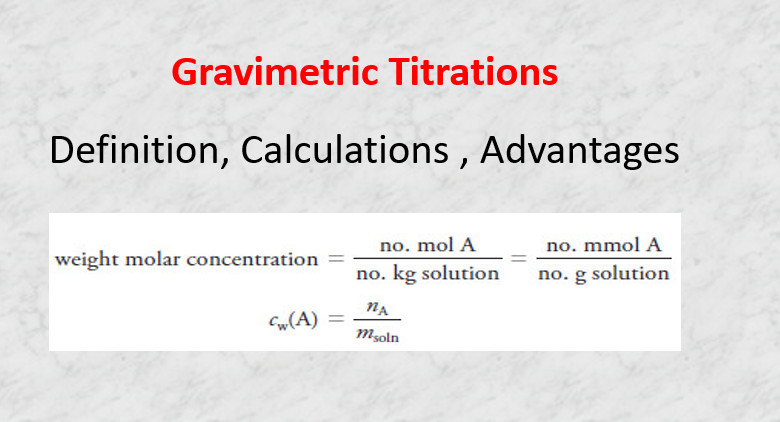
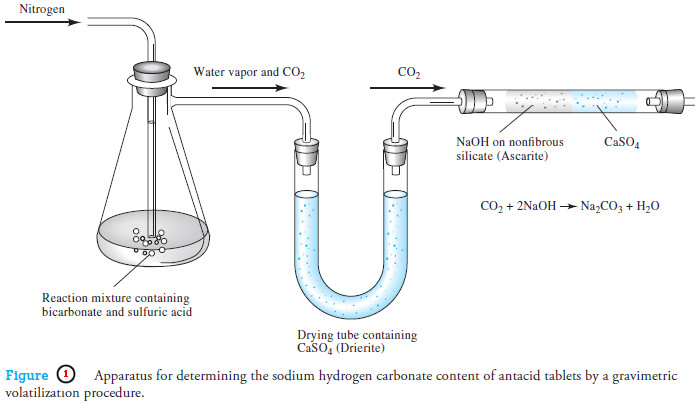
Gravimetric Titrations | Definition, Calculations & Advantages
Gravimetric titrations – Mass (weight) or gravimetric titrations differ from their volumetric counterparts in that the mass of titrant is…
Read More » -
Organic Chemistry
Areas of the Peaks in NMR Spectroscopy
– In this topic, we will discuss The Areas of the Peaks in NMR Spectroscopy Areas of the Peaks –…
Read More » -
Organic Chemistry
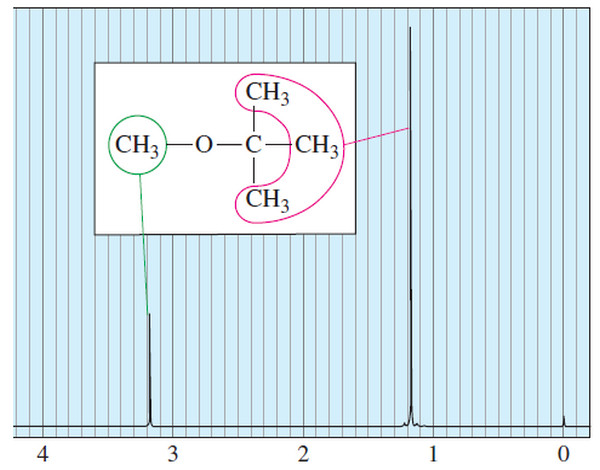
Number of Signals in NMR Spectroscopy
The Number of Signals – In general, the number of NMR signals corresponds to the number of different kinds of…
Read More » -
Physical Chemistry
Colloids: Definition, History and Types
– In this topic, we will discuss the colloids: definition, History and Types History of colloids – Thomas Graham (1861)…
Read More » -
Analytical Chemistry
Some Terms Used in Volumetric Titration
Some Terms Used in Volumetric Titration – A standard solution (or a standard titrant) is a reagent of known concentration…
Read More » -
Analytical Chemistry
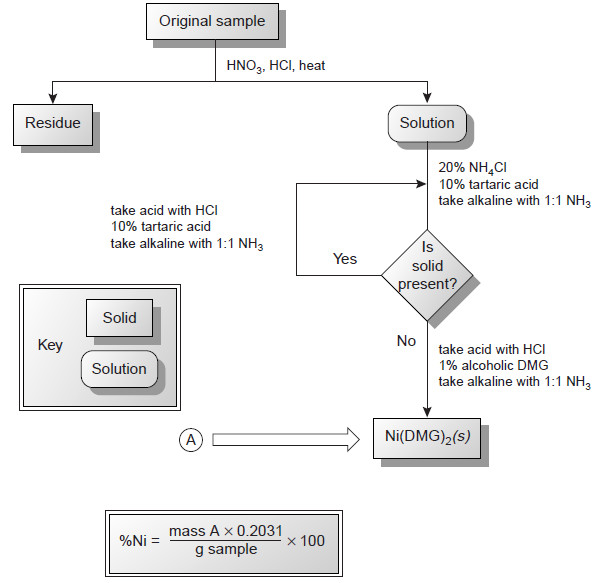
Applications of Gravimetric methods
Applications of Gravimetric methods – Gravimetric methods have been developed for most inorganic anions and cations, as well as for…
Read More » -
Organic Chemistry
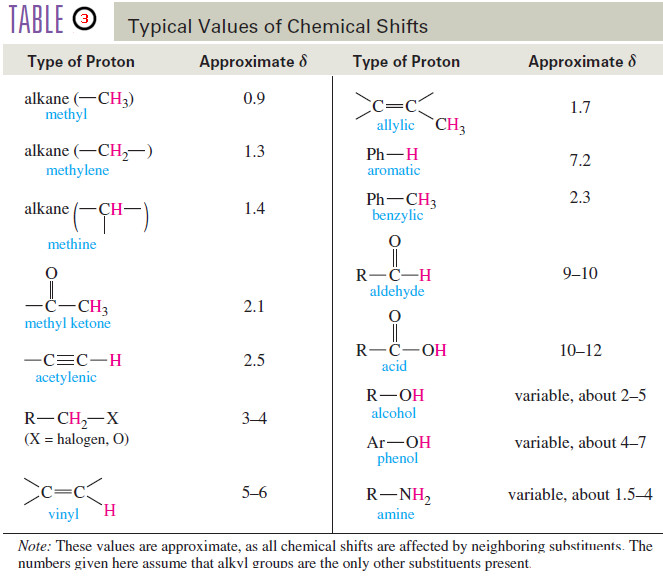
Chemical Shift in NMR Spectroscopy
– In this topic, we will discuss the Chemical Shift in 1H NMR Spectroscopy. What is Chemical Shift? – The…
Read More » -
Analytical Chemistry
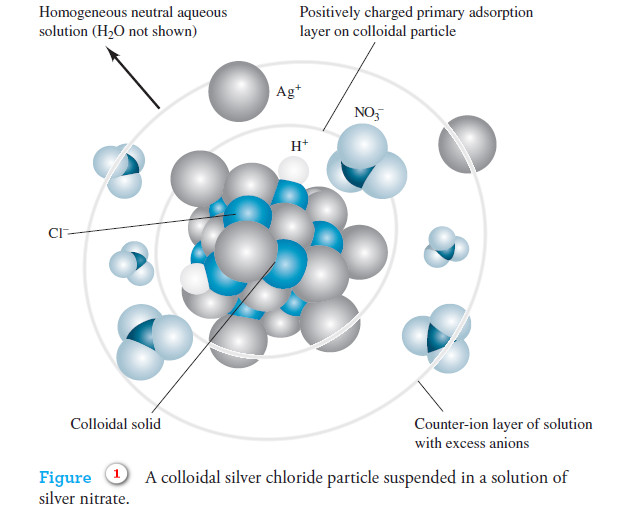
Precipitation Gravimetry
– In this topic, we will discuss the Precipitation Gravimetry as an important one of Gravimetric Analysis in analytical chemistry.…
Read More » -
Analytical Chemistry
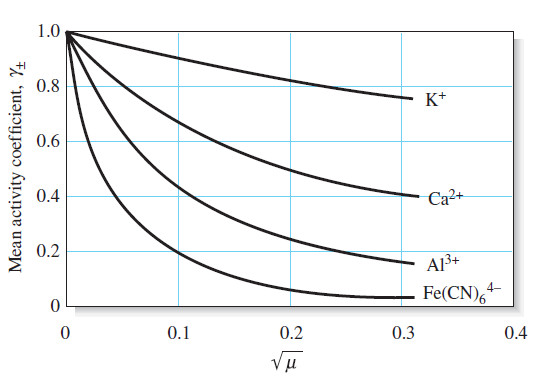
Activity Coefficients : Definition, Equation, Examples, Properties
– In this topic, we will discuss Activity coefficients : Definition, Equation, Examples and Properties. Activity Coefficients – Chemists use…
Read More » -
Analytical Chemistry
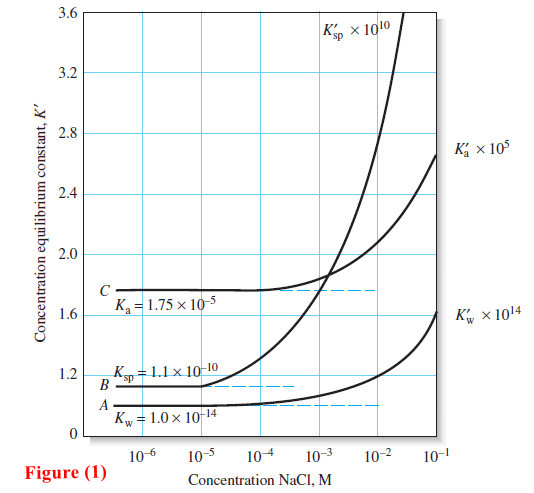
The Effect of Electrolyte on Chemical Equilibria
The Effect of Electrolyte on Chemical Equilibria – Experimentally, we find that the position of most solution equilibria depends on…
Read More » -
Analytical Chemistry
What is Analytical Chemistry?
What is Analytical Chemistry? Analytical chemistry is what analytical chemists do. – Analytical chemistry is too broad and active a…
Read More »